Abstract
Although very low birth weight infants are subjected to severe stress and glutamine is now considered a conditionally essential amino acid that may attenuate stress-induced protein wasting in adults, current amino acid solutions designed for neonatal parenteral nutrition do not contain glutamine. To determine whether a short-term supplementation with i.v. glutamine would affect protein metabolism in very low birth weight infants, 13 preterm neonates (gestational age, 28–30 wk; birth weight, 820–1610 g) receiving parenteral nutrition supplying 1.5 g · kg−-1 · d−1 amino acids and approximately 60 nonprotein kcal · kg−1 · d−1 were randomized to receive an i.v. supplement made of either 1) natural l-glutamine (0.5 g · kg−1 · d−1; glutamine group), or 2) an isonitrogenous glutamine-free amino acid mixture (control group), for 24 h starting on the third day of life. On the fourth day of life, they received a 2-h infusion of NaH13CO3 to assess the recovery of 13C in breath, immediately followed by a 3-h l-[1-13C]leucine infusion. Plasma ammonia did not differ between the groups. Glutamine supplementation was associated with 1) higher plasma glutamine (629 ± 94 versus 503 ± 83 μM, mean ± SD;p < 0.05, one-tailed unpaired t test), 2) lower rates of leucine release from protein breakdown (−16%, p < 0.05) and leucine oxidation (−35%, p < 0.05), 3) a lower rate of nonoxidative leucine disposal, an index of protein synthesis (−20%, p < 0.05), and 4) no change in protein balance (nonoxidative leucine disposal − leucine release from protein breakdown, NS). We conclude that although parenteral glutamine failed to enhance rates of protein synthesis, glutamine may have an acute protein-sparing effect, as it suppressed leucine oxidation and protein breakdown, in parenterally fed very low birth weight infants.
Similar content being viewed by others
Main
During the last decade, several clinical trials have produced compelling evidence for a role of glutamine in improving nitrogen balance in adult patients undergoing elective gastrointestinal surgery (1, 2), cholecystectomy (3), or bone marrow transplantation (4). Other studies suggested glutamine might exert a trophic effect on gut as well (5–7).
Although preterm infants are exposed to stress-induced protein wasting and intestinal frailty, conventional amino acid solutions designed for neonatal i.v. nutrition do not contain glutamine, because glutamine is classified as nonessential, has relatively poor solubility, and cannot be heat sterilized. Yet Neu et al.(8) recently showed that, compared with control enteral regimens, a 20-d supplementation of enteral feeding with glutamine (0.3 g · kg−1 · d−1) was safe, decreased the incidence of sepsis, and shortened hospital stay, as well as improved tolerance to subsequent enteral feeding, in preterm neonates. The regimens were, however, not isonitrogenous as the glutamine regimen provided more total nitrogen. Similarly, Lacey et al.(9) observed that i.v. glutamine facilitated weaning from parenteral nutrition and ventilatory support in a subgroup of infants with a birth weight <800 g. However, the decrease in the incidence of sepsis and hospital stay was not significant.
Because protein wasting impairs immune and respiratory function (10), one of the mechanisms for the putative clinical benefits of glutamine in VLBW infants could be through preservation of body protein. Enteral glutamine infusion was indeed associated with a drop in Ox in healthy adults—whether in the fasting state or made slightly catabolic by a glucocorticoid treatment (11, 12) —a rise in NOLD, an index of whole body protein synthesis in healthy adults (11), and a reduction in both Ox and protein breakdown in patients with Duchenne muscular dystrophy, a condition associated with severe, relentless muscle protein wasting (13).
The aim of this study was therefore to determine whether parenteral glutamine supplementation would acutely attenuate protein wasting in parenterally fed VLBW infants.
METHODS
Chemicals.
l-[1-13C]leucine, NaH13CO3 (both 99% C), and l-[2-15N]glutamine (99% N) were purchased from Cambridge Isotope Laboratories, (Andover, MA, U.S.A.). l-[1-13C]leucine and NaH13CO3 solutions were prepared in sterile 0.9% saline solution by the hospital pharmacy under a laminar flow hood and verified to be sterile (plate culture) and pyrogen-free by the Institut Départemental d'Analyze et de Conseil (IDAC, Nantes, France). Infusates were passed through a 0.22-μm Millipore filter (Bedford, MA, U.S.A.) and stored in sterile containers at 4°C until used. The concentrations of l-[1-13C]leucine and NaH13CO3 in the infused solutions were found to remain stable for >6 mo, as assessed by GCMS and GC-IRMS, respectively.
Natural l-glutamine (tissue culture grade) was obtained from Sigma Chemical Co. (Saint Quentin Fallavier, France), and verified to be pyrogen-free (rabbit temperature). Solutions of natural l-glutamine were prepared under sterile conditions in a laminar flow hood by the hospital pharmacy, filtered through a 0.22-μm filter, and stored in sealed glass containers at 4°C until used. The concentration of natural l-glutamine in the infusates was proven to be stable for 4 wk at 4°C.
Ion-exchange resins were obtained from Touzart et Matignon (Temex 1X8, 100–200 mesh hydrogen form, Courtaboeuf, France) and Aldrich (Dowex 50WX8–200, Saint Quentin Fallavier, France). All chemicals and isotopic purities were verified by GCMS. All other chemical reagents were from Sigma Chemical Co.
Patients.
Thirteen premature neonates weighting between 820 and 1650 g were recruited on the first day of life among babies admitted to the Neonatal Intensive Care Unit at the Hospital Mère et Enfant, Nantes, France. Written, informed consent was obtained from the parents before enrollment and after the purpose and potential risks of the study had been fully explained to them, according to procedures approved by the Ethical Committee of the University Hospital of Nantes, France (CCPPRB n°2, Région des Pays de la Loire). Inclusion criteria consisted of 1) birth weight between 700 g and 1650 g, 2) absence of sepsis, as judged from a C-reactive protein <20 mg/L, 3) mild lung disease, as judged from an oxygen requirement Fio2 <40%, and 4) normal plasma ammonia. Patients were excluded if they received or had received a blood transfusion or albumin infusion, if they had major surgery, had an elevated C-reactive protein concentration, required an Fio2 >40%, or if their parents refused participation in the study.
Nutritional regimens.
In both groups, parenteral nutrition was started on the first day of life. Glucose was started at approximately 5 mg · kg−1 · min−1 (approximately 7 g · kg−1 · d−1) and rapidly advanced as tolerated up to approximately 11 g · kg−1 · d−1. Parenteral amino acids (Primène 10%; Baxter/Clintec, Maurepas, France) were started on the first day at 0.5 g · kg−1 · d−1 and increased by 0.5 g · kg−1 · d−1 to reach 1.5 g · kg−1 · d−1 by the third day. Composition of the amino acid mixture is given in Table 1. Parenteral long-chain triglyceride emulsions (Ivelip 20%; Baxter/Clintec) were started on the second day of life at 1 g · kg−1 · d−1 and increased by 1 g · kg−1 · d−1 to reach 2 g · kg−1 · d−1 by the third day of life. None of the infants received any enteral nutrition until completion of the isotope infusion study on the fourth day of life.
In a double-blind fashion, patients were randomly assigned to receive either a 0.5 g · kg−1 · d−1 l-glutamine supplementation, provided as a 2.6 g/dL solution of l-glutamine in water (Sigma Chemical Co., St. Louis, MO, U.S.A.), or an isonitrogenous amino acid solution (Primène 10%, diluted to provide 3.3 g amino acid/dL). The supplementation started at 1800 h the third day of life, and was infused continuously by means of a calibrated syringe pump, and piggybacked into the i.v. central catheter line at a constant rate until 1600 h the next day.
Protocol design.
The isotopic study was performed on the fourth day of life in a total of 13 infants (six in the glutamine group, seven in the control group) while infants received continuous i.v. nutrition through a central venous catheter. The glutamine or Primène supplement was administered in a separate syringe, and piggybacked into the parenteral nutrition line, to be infused through the same central venous catheter.
At 1000 h on the isotope study day, measurement of respiratory gas exchange was started and continued throughout the study until 1600 h by means of an indirect calorimeter as described previously (14). At 1030 h, a baseline venous or capillary blood sample (0.5 mL) was obtained for measurement of background isotopic enrichment in plasma KIC. Three aliquots of expired air were obtained at 10-min intervals for determination of background 13CO2. For babies who were receiving ventilatory assistance, expired air was collected from the exhaust of the ventilator into a 10-L Douglas bag. For babies who were breathing spontaneously or with continuous positive airway pressure, expired air was collected from the outlet of the ventilated canopy. Triplicate aliquots of expired air from each sampling time were then immediately transferred with a syringe into airtight tubes (Exetainer system and gas testing vials, Labco, Bucks, U.K.) for later analysis.
Two stable isotope infusions were carried out consecutively on the same day in each infant through the central venous catheter (Fig. 1). First, a primed, continuous 2-h infusion of NaH13CO3 (7.5 μmol · kg−1 prime, 5 μmol · kg−1 · h−1 infusion) was performed from 1100 h to 1300 h. The purpose of this first isotope infusion was to estimate the rate of total Vco2. The labeled bicarbonate infusion was immediately followed by a primed, continuous 3-h infusion (approximately 48 μmol · kg−1 prime; approximately 48 μmol · kg−1 · h−1) of l-[1-13C]leucine from 1300 h to 1600 h, designed to assess leucine kinetics.
Venous or capillary blood samples (0.5 mL) were obtained to determine the concentration and 13C-enrichment of plasma KIC, and plasma leucine and glutamine concentrations at 30-min intervals during the last hour of the labeled leucine infusion. The total volume of blood sampled was therefore approximately 2 mL, which represents <5% of blood volume in a 1000-g infant. Expired air samples were obtained at 15-min intervals between 1200 h and 1300 h and between 1500 h and 1600 h, during the last hour of the labeled bicarbonate and labeled leucine infusions, respectively.
Analytical methods.
Known amounts of ketocaproic acid and l-[2-15N]glutamine were spiked into a 100-μL aliquot of plasma to serve as internal standards for the measurement of KIC and glutamine concentrations, respectively, by reverse isotope dilution. Plasma glutamine was isolated and derivatized as described (15). KIC was extracted from 100 μL of plasma by passing the acidified plasma sample over an AG50 cation-exchange column, and converted to its oxime-t-butyldimethylsilyl KIC derivative as described (16, 17). After derivatization, samples were then measured by GCMS.
GCMS assays.
The isotopic enrichment of KIC was determined using electron ionization GCMS on a 5890 series II gas chromatograph coupled with a 5971A mass selective detector (Hewlett Packard, Palo Alto, CA, U.S.A.) equipped with a capillary column (DB-1, 30 m × 0.25 mm ID, 0.25-mm film thickness, J&W Scientific, Courtaboeuf, France). Selected ion monitoring was used on ions of mass-to-charge ratio (m/z) 316 and 317, representing ions of the natural and [1-13C]KIC, respectively. An m/z 316 ion was also measured for ketocaproic acid. For glutamine analysis, ions at m/z 340 and 341 representing natural and [15N]-glutamine were selectively monitored.
Expired air assays.
E13co2 was measured by GC-IRMS (Finnigan-MAT, Bremen, Germany) using a Porapak column.
Steady state.
A measurement was considered to be in steady state when its coefficient of variation was <10% during the period considered.
Calculations of leucine kinetics.
As shown by earlier studies (18), 13C-labeled bicarbonate infusion can be use to estimate the Raco2, which was calculated as follows:
Eq. 1

where ibicarb is the labeled bicarbonate infusion rate (μmol · kg−1 · h−1), and Eibicarb and EbicarbCO2 are the 13C enrichments in the labeled bicarbonate infusate and expired air during the last hour of labeled bicarbonate, respectively.
Leucine appearance (RaLeu in μmol · kg−1 · h−1) into the plasma compartment was calculated as
Eq. 2

where iLeu is the rate of 13C-leucine infusion (μmol · kg−1 · h−1), and EiLeu and EpKIC are the 13C enrichments (mole percent excess) in the infusate leucine and in plasma KIC at steady state, respectively. Because leucine kinetics were measured under fed conditions, both exogenous leucine from parenteral nutrition (PNLeu) and endogenous leucine contributed to the appearance rate of leucine. Furthermore, because leucine is an essential amino acid, release from protein breakdown (BLeu) is its only endogenous source. So, protein breakdown can be calculated as
Eq. 3

In the control group, PNLeu included the leucine supplied by the diluted amino acid supplement.
The appearance of 13CO2 in expired air (F13co2, in μmol · kg−1 · h−1) is calculated as
Eq. 4

where ELeuCO2 is the 13CO2 enrichment at steady state during the [13C]leucine infusion. Leucine oxidation (OxLeu, in μmol · kg−1 · h−1) was determined by the following equation:
Eq. 5

This rate of leucine oxidation represents total oxidation, and includes the oxidation of labeled leucine as well.
NOLD, an index of whole body protein synthesis, was calculated as
Eq. 6

This rate of NOLD includes the nonoxidative disposal of labeled leucine as well.
Finally, net leucine balance, an index of the net protein leucine gain, was calculated as
Eq. 7

Under conditions of steady state, leucine balance also equals the difference between total leucine intake—both natural leucine from parenteral nutrition and labeled leucine—and total leucine irreversible loss (OxLeu):
Eq. 8

Sample size.
In earlier studies in adults (11), enteral glutamine administration was associated with a 30– 40% decline (δ) in OxLeu—19.0 versus 11.0 μmol · kg−1 · h−1—whereas the SD (ς) of OxLeu was 5.3 μmol · kg−1 · h−1, so that δ/ς = (19 − 11) / 5.3 = 1.5. Assuming a similar glutamine-induced change in OxLeu in the infants to be enrolled in the current study, the number of patients necessary was estimated as follows:n = (Zα +Zβ)2 / (δ2 / ς2), where Zα = 1.96, and Zβ = 0.84 with an α < 0.05, and a power (1 − β) of 80% [using the tables in Friedman et al.(19)]. It follows that n = (1.96 + 0.84)2 / (1.5)2 = 7.84 / 2.25 = 3.5, for a one-tailed test. Our goal was therefore to recruit five to seven subjects in each group.
Statistics.
Results are expressed as mean ± SD. Comparisons between groups were performed by using one-tailed unpaired t test; significance was established at p < 0.05.
RESULTS
Selected relevant clinical characteristics for the studied population, as well as the babies' nutritional intake on the day of the isotopic study (d 4), are given in Table 2. There was no significant difference between the two groups for birth weight or gestational age, even though the term was slightly, but not significantly greater in the control group, as there were more growth-retarded newborns in the control group (3 versus 1). The average postnatal age at the time of the isotope infusion study was not statistically different between the groups (70 ± 4 h and 74 ± 7 h in the glutamine and control groups, respectively). Weight loss between birth and isotope infusion day was similar between groups (103 ± 34 g versus 105 ± 46 g, i.e. 9.7 ± 3.4%versus 8.8 ± 3.1% of birth weight; NS).
Three infants received caffeine in the glutamine group, one infant in the control group received insulin, and another one in the same group received midazolam (Hypnovel). None of these newborns received dopamine. With regard to antenatal corticoid treatment, two of the six infants in the glutamine group had received one or two courses of treatment, compared with five of seven in the control group.
Regarding ventilatory status, some infants were breathing spontaneously, whereas others received mechanical ventilation, including ventilation with nasal continuous positive airway pressure. None of the infants received an Fio2 >40%. The average Fio2 was 23 ± 3% in the glutamine group and 25 ± 2% in the control group.
Plasma glutamine concentration was higher in the glutamine group (629 ± 94 μM versus 503 ± 83 μM, p < 0.05, one-tail t test). Serum ammonia was not significantly different in the two groups: 79 ± 16 μM in the glutamine group versus 69 ± 31 μM in the control group.
Steady-state conditions, as defined by coefficients of variation <10%, were obtained during the last hour of tracer infusion for plasma KIC concentration, [13C]KIC and [13]CO2 enrichments (Fig. 2), therefore allowing the use of steady-state equations for calculation of leucine kinetics. Plasma KIC concentrations were not different between the glutamine group and the control group (25 ± 10 μM versus 28 ± 6 μM). Raco2, measured by using labeled bicarbonate infusion, was not different between the two groups: 7.6 ± 2.0 mL · kg−1 · min−1 in the glutamine group versus 8.7 ± 2.5 mL · kg−1 · min−1 in the control group.
RaLeu was approximately 24% lower (p < 0.01) and BLeu was reduced by approximately 17% in the glutamine group (p < 0.05;Table 3), compared with controls. Glutamine infusion was associated with an approximately 35% reduction in OxLeu as well (p < 0.05). Yet NOLD, an index of whole-body protein synthesis, was approximately 20% lower in the glutamine group, compared with the control group (p < 0.05). Net leucine balance, defined as the difference between total leucine intake and total leucine irreversible loss (OxLeu), was positive in five of six infants in each group, and was therefore significantly different from zero in each group. Net leucine balance did not differ between the two groups.
DISCUSSION
The specific aim of the current study was to determine whether a short, i.v. glutamine infusion, compared with an isonitrogenous amino acid mixture, was able to acutely affect protein metabolism in parenterally fed VLBW infants in the first few days of life. Our findings suggest that glutamine acutely suppressed rates of protein breakdown and leucine oxidation, but decreased rates of NOLD, an index of whole body protein synthesis, while both groups were receiving a total amino acid intake of 2 g · kg−1 · d−1. Further studies would be required to determine whether more prolonged glutamine administration, along with higher protein intakes, or in more acutely ill preterm infants, would enhance overall protein accretion in VLBW infants.
Even though glutamine was only administered for <24 h, significant changes in leucine kinetics were detectable in the glutamine-supplemented group during the last 3 h of glutamine infusion, compared with the group receiving an isonitrogenous control amino acid mixture. The change observed in OxLeu (−35%) is of the same magnitude as reported in other populations. For instance, oral glutamine decreased OxLeu by approximately 37% in healthy adult volunteers (11), approximately 26% in adult volunteers made slightly catabolic by prednisone treatment (12), and approximately 35% in 8– to 13-y-old children suffering from Duchenne muscular dystrophy, a chronic disease characterized by chronic, relentless muscle protein wasting (13). Whereas the latter two studies were conducted in the fasted state, the current study is the first to demonstrate that glutamine can suppress OxLeu in the fed state as well.
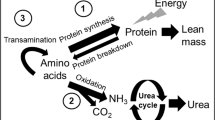
The mechanism for the glutamine-induced decline in OxLeu remains to be elucidated. A decline in OxLeu may either be 1) as a consequence of an increase in protein synthesis or 2) as a consequence of direct inhibitory effect of glutamine on OxLeu.
A rise in protein synthesis was not detectable in the current study, as NOLD was in fact lower in the glutamine-supplemented group. Inasmuch as NOLD reflects the summation of the synthetic rates of a host of different proteins in various organs, this finding does not, however, rule out a putative stimulation of protein synthesis by glutamine in specific tissues. For instance, if skeletal muscle is a prime target of the putative protein anabolic effect of glutamine (1), the fact that skeletal muscle accounts for a much smaller fraction of body protein stores in VLBW infants, compared with adults, may explain why an increase in NOLD was not detected.
Alternatively, glutamine may primarily inhibit OxLeu rather than enhance protein synthesis. Although OxLeu is known to be tightly dependent on circulating leucine concentration (20), and although plasma leucine concentrations were not measured in the current study, the reduction in OxLeu is unlikely to result from a lower plasma leucine concentration: plasma KIC concentrations indeed were not different between groups (25 ± 10 μM versus 28 ± 6 μM), and plasma leucine usually correlates well with plasma KIC levels. Alternatively, glutamine can be readily used as a source of energy, because its conversion by glutaminase and glutamate dehydrogenase leads to α-ketoglutarate, which can, in turn, be oxidized in the tricarboxylic acid cycle. Glutamine is indeed known to be a preferred source of energy for rapidly dividing cells (21). We speculate that enhanced glutamine availability may increase glutamine oxidation and produce NADH, thus affecting cellular redox status. Changes in redox status are known to alter OxLeu through inhibition of branched-chain keto acid dehydrogenase, a key enzyme in leucine catabolism: for instance long-chain FFA, another prominent source of energy, are known to inhibit branched-chain keto acid dehydrogenase (22, 23).
In the current study, glutamine supplementation was associated with a significant reduction in BLeu as well. This result is consistent with our earlier studies: in another group of VLBW infants, enteral glutamine delivery was associated with a trend toward a reduction in BLeu; in that previous study, the approximately 16% decline, however, failed to reach statistical significance (24). Besides a type II statistical error, several differences in study design can, however, account for the difference: in that earlier study, glutamine was supplied via the enteral route, and the dose was only 40% of that used in the current study (0.2 versus 0.5 g · kg−1 · d−1). In earlier studies in adults, glutamine did not alter rates of proteolysis (11), yet oral glutamine was associated with an approximately 8% decline in proteolysis in children with muscular dystrophy (13). The mechanism for this effect remains to be found. Because insulin inhibits proteolysis in a dose-dependent fashion in adult humans (25), glutamine may, in theory, suppress proteolysis through enhanced insulin secretion. This is, however, unlikely as 1) proteolysis seems to be somehow insulin resistant in neonates (26), and amino acids may be the main factor regulating proteolysis in the neonatal period; and 2) moreover, although insulin levels were not measured in the present study, we failed to observe any rise in insulin secretion in healthy adults given large oral doses of glutamine in previous studies [(11), and Darmaun et al. unpublished personal results]. In earlier studies, we found glutamine utilization rate and metabolic clearance rate (glutamine utilization rate divided by plasma glutamine concentration) to be much higher in VLBW infants than older children (24). The higher clearance rate of plasma glutamine may point to a greater glutamine requirement in VLBW infants. It is tempting to speculate that lack of sufficient glutamine availability in the VLBW infants receiving glutamine-free amino acid solutions may lead to enhanced rates of protein breakdown to produce the larger amounts of glutamine needed by fast-growing tissues. The acute reduction in rates of protein breakdown observed on i.v. glutamine infusion in the current study is compatible with the latter hypothesis.
Glutamine supplementation failed to enhance NOLD, an index of protein synthesis, in the current study, and was in fact even associated with a reduction in NOLD (Fig. 3). Although this contrasts with the protein anabolic effect of glutamine observed in healthy adults (11), failure of glutamine to enhance NOLD was, in fact, observed in prednisone-treated adults (12) and children with Duchenne muscular dystrophy (13) as well. In all three clinical situations, insufficient amino acid availability may preclude the protein anabolic effect of glutamine as 1) our earlier studies (12, 13) were conducted in fasting subjects, and 2) in the current study, our patients only received a total amino acid intake of 2 g · kg−1 · d−1 on the fourth day of life, and would have reached their optimal intake of 3–3.5 g · kg−1 · d−1 by d 6. This is because the introduction of amino acids has traditionally been prudent in the first day of life in VLBW infants, and amino acid intake gradually advanced thereafter, owing to concerns about the ability to metabolize amino acids with the putative risk of eliciting supraphysiologic elevations of plasma amino acid concentrations or blood urea nitrogen, and metabolic acidosis. Such a prudent approach may not be warranted as a recent study (27) suggests that 3 g · kg−1 · d−1 parenteral amino acid intake from the first day of life may be well tolerated and associated with significantly greater rates of protein accretion. Further studies would be required to determine whether the same dose of glutamine, supplied for a longer time, and along with a more generous amino acid intake (e.g. 3 g · kg−1 · d−1), would enhance rates of whole body protein synthesis.
In conclusion, although glutamine supplementation failed to enhance estimates of protein synthesis, i.v. glutamine may preserve body protein as it acutely suppressed rates of OxLeu and BLeu in VLBW infants on the fourth day of life. Further studies would be warranted to determine whether prolonged glutamine infusions with a more generous protein intake would promote protein accretion in this patient population.
Abbreviations
- BLeu:
-
leucine release from protein breakdown
- Eco2:
-
13CO2 enrichment
- EiLeu:
-
isotopic enrichment in infusate 13C-leucine
- EpKIC:
-
isotopic enrichment in plasma KIC at steady state
- F13co2:
-
appearance of 13CO2 in expired air
- Fio2:
-
inspired air oxygen fraction
- GC-IRMS:
-
gas chromatography-isotope ratio mass spectrometry
- GCMS:
-
gas chromatography-mass spectrometry
- ibicarb:
-
rate of [13C]bicarbonate infusion
- iLeu:
-
rate of [13C]leucine infusion
- KIC:
-
α-keto-isocaproate
- NOLD:
-
nonoxidative leucine disposal
- OxLeu:
-
leucine oxidation
- RaLeu:
-
appearance rate of leucine into plasma
- IUGR:
-
intrauterine growth retardation
- Vco2:
-
CO2 production
- VLBW:
-
very low birth weight
- PNLeu:
-
exogenous leucine from parenteral nutrition
References
Hammarqvist F, Wernerman J, Ali R, Von der Decken A, Vinnars E 1989 Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg 209: 455–461
Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lawin P, Furst P 1989 Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet 1: 231–233
Hammarqvist F, Wernerman J, Von der Decken A, Vinnars E 1990 Alanyl-glutamine counteracts the depletion of free glutamine and the postoperative decline in protein synthesis in skeletal muscle. Ann Surg 212: 637–645
Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortos K, Bye R, Morrow FD, Jacobs DO, Smith RJ, Antin JH, Wilmore DW 1992 Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation: a randomized, double-blind, controlled study. Ann Intern Med 116: 821–828
Scheppach W, Loges C, Bartman P, Christl S, Richter F, Dusel G, Stehle P, Furst P, Kaspar H 1994 Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the ileum and colon. Gastroenterology 107: 429–434
Tremel H, Kienle B, Weileman L, Stehle P, Furst P 1994 Glutamine dipeptide-supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology 107: 1595–1601
Van Der Hulst R, Van Krell B, Von Meyenfeldt M, Brummer R, Arends J, Deutz N, Soeters P 1993 Glutamine and the preservation of gut integrity. Lancet 334: 1363–1365
Neu J, Roig J, Meetze W, Veerman M, Carter C, Millsaps M, Bowling D, Dallas M, Sleasman J, Knight T, Auestad N 1997 Enteral glutamine supplementation for very low birth weight infants decreases morbidity. J Pediatr 131: 691–699
Lacey J, Crouch J, Benfell K, Ringer S, Wilmore C, Maguire D, Wilmore D 1996 The effects of glutamine-supplemented parenteral nutrition in premature infants. JPEN J Parenter Enteral Nutr 20: 74–80
Chandra R 1983 Nutrition, immunity, and infection: present knowledge and future directions. Lancet 26: 688–691
Hankard R, Haymond M, Darmaun D 1996 Effect of glutamine on leucine metabolism in humans. Am J Physiol 271: E748–E754
Darmaun D, Welch S, Sager B, Altomare A, Haymond M 1998 Can glutamine alter the protein catabolic response to prednisone in humans?. Clin Nutr 17: 62( abstr)
Hankard R, Hammond D, Haymond M, Darmaun D 1998 Oral glutamine slows down whole body protein breakdown in Duchenne muscular dystrophy. Pediatr Res 43: 222–226
Rozé J, Chambille B, Dehan M, Gaultier C 1994 Measurement of oxygen uptake in newborn infants during assisted and spontaneous ventilation. Respir Physiol 98: 227–233
Darmaun D, Manary M, Matthews D 1985 A method for measuring both glutamine and glutamate levels and stable isotopic enrichments. Anal Biochem 147: 92–102
Salman EK, Haymond M, Bayne E, Sager BK, Wiisanen A, Pitel P, Darmaun D 1996 Protein and energy metabolism in prepubertal children with sickle cell anemia. Pediatr Res 40: 34–40
Liet J, Piloquet H, Marchini J, Maugère P, Bobin C, Rozé J, Darmaun D 1999 Leucine metabolism in preterm infants receiving parenteral nutrition with medium-chain compared with long-chain triacylglycerol emulsions. Am J Clin Nutr 69: 539–543
Spear M, Darmaun D, Sager B, Parsons W, Haymond M 1995 Use of [13C]-bicarbonate infusion for measurement of CO2 production. Am J Physiol 268: E1123–E1127
Friedman LM, Furberg CD, De Mets DL (eds) 1983 Sample size. In: Fundamentals of Clinical Trials. John Wright, Boston, pp 69–88
Goulet O, DePotter S, Salas J, Robert J, Rongier M, Ben Hariz M, Koziet J, Desjeux J, Ricour C, Darmaun D 1993 Leucine metabolism at graded amino acid intakes in children receiving parenteral nutrition. Am J Physiol 265: E540–E546
Souba W 1991 Glutamine: a key substrate for the splanchnic bed. Annu Rev Nutr 11: 285–308
Buxton D, Olson M, Taylor M, Barrob L 1984 Regulatory effects of fatty acids on decarboxylation of leucine and 4-methyl-2-oxopentanoate in the perfused rat heart. Biochem J 221: 593–599
Wagenmakers A, Veerkamp J 1984 Degradation of branched-chain amino acids and their derived 2-oxo acids and fatty acids in humans and rat heart and skeletal muscle. Biochem Med 28: 16–31
Darmaun D, Roig J, Auestad N, Sager B, Neu J 1997 Glutamine metabolism in very low birth weight infants. Pediatr Res 41: 391–396
Fukagawa N, Minaker K, Rowe J, Goodman M, Matthews D, Bier D, Young V 1985 Insulin-mediated reduction of whole body protein breakdown: dose-response effects on leucine metabolism in post-absorptive men. J Clin Invest 76: 2306–2311
Poindexter B, Karn C, Ahlrichs J, Wang J, Leitch C, Liechty E, Denne S 1997 Amino acids suppress proteolysis independent of insulin throughout the neonatal period. Am J Physiol 272: E592–E599
Paisley J, Thureen P, Baron K, Hay W 2000 Safety and efficacy of low versus high parenteral amino acids intakes in extremely low birth weight neonates immediately after birth. Pediatr Res 47: 293A( abstr)
Acknowledgements
The authors thank the parents of the infants who agreed to participate in this study, and the nurses of the Neonatology Unit for their superb help. We also thank Pascale Maugère and Odile Desfontaines for their excellent technical help, and Dr Philippe Mauran, Dr Isabelle Falconi, and Professor Françoise Ballereau of the Pharmacie Hôtel-Dieu, Nantes, for the preparation of the stable isotope solutions and natural glutamine solutions for i.v. administration.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by a Programme Hospitalier de Recherche Clinique (PHRC) grant from the Ministry of Health, Paris, France, and the Délégation à la Recherche Clinique, CHU de Nantes. C.d.R. was supported, in part, by fellowship grants from the Fondation pour la Recherche Médicale (FRM), France, and the IPSEN Laboratories, France. O.L.B. was supported by a fellowship grant from the Ministère de la Recherche, France.
Rights and permissions
About this article
Cite this article
Des Robert, C., Le Bacquer, O., Piloquet, H. et al. Acute Effects of Intravenous Glutamine Supplementation on Protein Metabolism in Very Low Birth Weight Infants: A Stable Isotope Study. Pediatr Res 51, 87–93 (2002). https://doi.org/10.1203/00006450-200201000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200201000-00016
This article is cited by
-
Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation
Nature Communications (2017)
-
δ15N and δ13C in hair from newborn infants and their mothers: a cohort study
Pediatric Research (2012)