Abstract
Heart rate (HR) acceleration is an essential mechanism for adaptation to changes in hemodynamic and energetic needs resulting from body movements. To evaluate age-related development of coupling between spontaneous movement and HR changes, we performed polysomnographic recordings in 20 clinically and neurologically normal newborns including 10 premature (31- to 36-wk gestational age, wGA) and 10 full-term (38- to 41-wk gestational age) infants. Recordings were sampled at 286 Hz and processed using a signal-to-noise ratio algorithm for QRS complex detection. Movements were automatically detected and the logical signal obtained was sampled at QRS fiducial points and written in the attributes of each QRS. The study included the 402 movements that were less than 30 s in duration and were neither preceded nor followed by another movement or by a respiratory event (pause, sigh). The amplitude of movement-induced HR acceleration was significantly lower in premature compared with full-term newborns (p < 0.01). This difference persisted when the other factors influencing the HR response (basal HR, movement duration, and amplitude) were taken into consideration. Our data identify HR acceleration induced by spontaneous body movements as a fundamental reflex response that develops with gestational age from premature to full-term newborns.
Similar content being viewed by others
Main
HR acceleration is an essential mechanism for adaptation to changes in hemodynamic and energetic needs resulting from BM. Exercise studies in adults demonstrated that cardiovascular response to movements is matched with increased oxygen consumption(1). Strong positive association between the increase in heart rate, in the energy expenditure, and in oxygen uptake have been described(2). Numerous studies have documented the mechanisms underlying motion-HR reflex coupling(3–6). HR fluctuations related to BMs involve both the parasympathetic and sympathetic components of the ANS(1,7–9).
HR changes related to spontaneous BMs occur consistently during sleep in normal adults, and their pattern, marked primarily by initial acceleration, varies with the type and duration of the movement(10–12). Decreased HR responses to BMs may be a useful early indicator of ANS damage(13–14). Erkenjuntti and Kero(15) consistently found HR acceleration in response to BMs in normal sleeping full-term newborns. Fetal HR changes (acceleration) in response to fetal movement increases with gestational age(16). However, to our knowledge, no data on the HR response to BMs in healthy premature as compared with full-term newborns are available.
Data from the literature and our previous investigations on HR variability in various frequency bands show that the tone of both ANS components increases with GA and is sleep state dependent(17–19). We hypothesized that the low vagal and sympathetic tone in premature infants may result in failure to produce an adequate HR response to BMs during sleep. We tested the coupling between HR and spontaneous BMs according to GA, sleep states, baseline HR, and BM characteristics in clinically and neurologically normal newborns with GAs of 31 to 41 wk.
SUBJECTS
The study was carried out in clinically and neurologically normal newborns with a postnatal age of 2 to 6 d. Ten infants were premature (31 to 36 wGA on the day of the recording) and ten were full-term (38 to 41 wGA). All infants were appropriate for GA, with birth weights between the 10th and 75th percentiles of reference curves for French newborns(20,21).
Age and normality of the infants were determined based on concordance of the following criteria: 1) first day of the mother's last menstrual period (only babies born to women with regular menstrual periods were included); 2) intrauterine ultrasonography parameters; 3) clinical and neurological data; 4) weight, body length, and head circumference at birth, and 5) EEG pattern(22). The Apgar score(23) at birth was ≥ 7 for premature and ≥ 9 for full-term infants. Each mother gave her consent to the study after receiving detailed explanations of recording methods and study objectives. The study was approved by our institution's ethics committee.
METHODS
Data recordings. Recordings were performed after feeding, in the maternity ward, from 0900 to 1300 h. Infants of 38-41 wGA wore normal clothes and lay in their own cots with room temperature of 25-26°C. Premature infants were in incubators at 30-34°C and the incubator temperature was adapted to maintain normal body temperature. All infants slept in the supine position.
Polygraphic recordings of spontaneous sleep episodes included monitoring of at least the centrooccipital, left, and right bipolar EEG channels, eye movements recorded by piezoelectric crystal transducer, thoracic and abdominal respiratory movements recorded by mercury strain gauges, air flow recorded by nasal and buccal thermistors, chin and diaphragmatic surface EMGs, ECG, cardiotachographic tracings, and binary clock signal, according to previously described methods(22). Transcutaneous PO2 was monitored continuously, and behavior was observed by two independent well-trained observers. Motor activity of both upper and lower limbs was recorded using piezoelectric crystal transducers attached to the dorsal surfaces of the hands and feet (see details inRefs. 22 and 24).
Data processing. Signals used for this study (ECG, body movements, abdominal respiratory movements, and nasal flow) were sampled at 286 Hz.
A signal-to-noise ratio algorithm was used to detect QRS complexes. A fiducial point in the QRS wave was localized. ECG artifacts were recognized automatically by RR intervals above or below predefined threshold and by large (>30%) differences from adjacent RR values(25), followed by visual inspection of all movement periods.
BM (with signal-to-noise ratio > 2) were undersampled at 9 Hz. A logical signal was created to test for the presence of movements of the upper and lower limbs, which were summed for the further analyses(26). Movements taken into account were automatically determinate as follows: 1) Only movements that lasted for 0.2 s or more were included into the study; 2) two movements separated by an interval of less than 1 s were considered to be the same movement. The logical signal was sampled at QRS fiducial points and written in the attributes of each QRS. Movements less than 30 s in duration that were neither preceded nor followed (within 10 s) by another movement were automatically selected. Each movement was visually inspected on the computer screen and it possible relations to respiratory pauses (respiratory signal <20% of the three previous breathings amplitude), sighs (abdominal respiratory signal amplitude ≥ twice the amplitude of the three preceding or after breathings), or startles (brief sudden movement confirmed by observation) were noted. The amplitude of each movement was assessed using a three-point scale as follows: small amplitude two to five times the background noise; 3) mid-range, 5 to 10 times the background noise; 4) high, >10 times the background noise. Movement amplitude was evaluated twice, at onset of and at the maximum of the movement. Movement duration was measured in milliseconds.
RR modification related to body movements was estimated based on values normalized for the mean basal RR interval duration (15 heart beats preceding the movement) as follows: RR modification = [(mean RR before the BM - minimum RR during the BM)/(mean RR before the BM) %]. Data obtained by this calculation will be designated hereafter by the abbreviation %RRM (percentage of RR modification).
Sleep states were defined visually based on the concordance of two criteria: 1) EEG patterns characteristic for each conceptional age and 2) rapid eye movements present during AS but absent during QS. Periods with a discrepancy between these two criteria were classified as IS (details inRef. 22). Because there were few periods of wakefulness without gross body movements and crying (inducing artifacts), and few interpretable IS periods of 1 min or more in duration, only AS and QS periods were considered for our study.
Statistics. Statistics presented hereafter were performed using ANOVA and regression analyses and were based on means per infant. In all cases, comparison of medians by Mann-Whitney and Wilcoxon tests confirmed ANOVA data.
RESULTS
We found 916 BMs of less than 30-sec duration. A total of 292 BMs were excluded after visual inspection because of ECG or other artifacts. Of the remaining 624 BM, 74 were rejected because they were associated with respiratory pauses, sighs, or startles that are known to modify HR(27,28). A total of 148 BMs that occurred during usually too short IS periods were eliminated. This left 402 BMs not preceded and not followed by another BM or a respiratory event and occurring during stable AS or QS periods. The mean number of BMs per infant was 19.9 ± 12.3 (range 5 to 51); each infant had BMs in both AS and QS. The majority of BMs included into the study (62.4%) occurred in AS. Visual inspection of the 402 BMs included in our analysis showed that they all occurred without any interruption (the possibility of interruption shorter than 1 s was included in the software).
On average, only 7.4% of BMs before 38 wGA and 2.8% beyond this age were accompanied by mild (less than 10 beats per min) HR deceleration. The majority of BMs induced HR acceleration, whose amplitude varied with GA (see bellow).
For all the BMs included in the analysis, HR returned to the basal level within 10 s after the BM. For many BMs of more than 5 s duration, the progressive heart rate deceleration after the initial acceleration started before BM termination. BMs of more than 10 s duration were sometimes associated with small RR interval fluctuations superimposed on the background RR decrease and increase.
We did not find significant correlations between mean movement duration and RR modifications observed (p > 0.4, r = 0.3). Relationships with visually evaluated BMs amplitude were also nonsignificant (for amplitude at BMs onset p > 0.9; for amplitude at the maximum of the BMs p > 0.2).
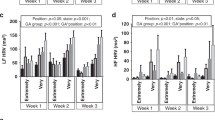
No significant between-sleep state difference was observed in 31-36 wGA (p > 0.7) and 38-41 wGA (p > 0.6) infants (Fig. 1). No significant interactions between age and sleep state factors were found.
Mean heart rate modifications (acceleration) related to body movements in healthy appropriate for gestational age premature 31-36 wk GA (prem) and full-term > 37 wk GA (term) newborns. ANOVA analysis is based on means per infant and per sleep states. Note that in AS as well as in QS mean %RRM was significantly lower in premature as compared with at term newborns (p < 0.002); no between-sleep state difference (NS) was observed in both ages.
The %RRM was significantly correlated with gestational age (p < 0.004, r = 0.71, Fig. 2). In total tracing, as well as in AS and in QS, mean %RRM was significantly lower in premature as compared with at term newborns (p < 0.002, Fig. 1).
Correlations between gestational age (on abscissa) and RR modifications after body movements during total AS and QS time. On ordinate: percentage of mean heart rate modifications normalized for baseline RR values using the formula: [(mean RR interval before movement - minimal RR interval during movement): mean RR interval before movement] %. Each point correspond to one (mean) infant data.
As specified in "Methods," RR modifications induced by BMs were normalized for basal RR interval duration. However, because both basal RR intervals(18,19) and %RRM are age dependent, we had to confirm that the age factor per se is able to determine the amplitude of HR acceleration induced by body movements. To this end, we studied BM-related HR modifications during 151 BMs preceded by short mean basal RR intervals (420 to 500 ms), including 90 in premature and 61 in full-term infants. Distribution of mean basal RR was similar in both groups (p > 0.6; correlation coefficient between age and basal RR, 0.08). Again, in this selected group of BMs with similar basal RR levels, HR acceleration was significantly greater in full-term than in premature newborns (p < 0.01).
DISCUSSION
Our data demonstrate that HR reactivity to spontaneous BMs was significantly correlated with gestational age. Premature infants showed little ability to accelerate their HR, whereas most BMs in infants born after 37 wGA were accompanied with marked HR acceleration.
The various markers for ANS maturation during early ontogenesis are closely linked. One of the crucial methodological questions in many ontogenetic and pathological situations is the intercorrelation between the basal heart rate and the amplitude of the spontaneous or induced heart rate modification. Our study demonstrated that age-related differences in the HR response were significant independently from the basal HR, which is normally faster in the premature than in the full-term infants(19). First, our analyses were based on the percentage of BM-related modifications normalized for the mean basal RR interval. In addition, the influence of age on HR acceleration amplitude (greater in full-term than in premature infants) was confirmed by the study of BMs occurring in periods with similar mean basal RR intervals in both premature and at term.
HR changes associated with both short and long BMs occurred rapidly, probably consistent with an withdrawal of the vagal decelerating component early during the BM. Data from the literature suggest that vagal activity is present very early during ontogenesis(29–31). In our study, the contrast between premature infants and more than 38 wGA infants was similar to the observed for high-frequency HR variability, which has been reported to be significantly higher at 37-38 wGA than at 31-34 and 35-36 wGA(32). Given that both high-frequency HR variability and BM-related HR acceleration are closely dependent on vagal control(1), we suggest that this contrast may reflect a significant step in parasympathetic ANS maturation occurring at about 36 wGA. On the contrary, changes in low-frequency HR variability, which is mainly under sympathetic control, have been reported to occur more gradually, with significant differences only between 31-34 wGA and 39-41 wGA infants(32). All these data agree with the studies of autonomic reflexes in 27-37 wGA newborns conducted by Lagercrantz et al.(33), which demonstrated a tendency toward a dominance of parasympathetic over sympathetic responses, especially in the older age groups studied.
It has been demonstrated that in infants, oxygen consumption is higher in AS, the state with increased body movements, compared with QS(24,34–35). Relationships between intensive motor activity or handling (accompanied by crying) and hypoxemia have been well documented(36–38). In our knowledge, there are no quantified studies on relationships among HR, body movements, and oxygen consumption in newborns. Our data demonstrate age-related modifications between two components of this trilogy: premature babies are less able to react to body movement by HR acceleration than full-term newborns and older subjects. Further studies are necessary to test potential relationships between this incapacity and the maintain of adequate oxygenation in premature babies.
Comparison of our data in newborns with those obtained in utero is difficult. In utero studies did not focus on isolated BMs (i.e. BMs neither preceded nor followed by other BMs), and investigated only long BMs (i.e., BMs lasting more than 5 s or more than 15 s(16,39)). Despite differences in age grouping, all these studies found that HR-BM coupling increased with gestational age and manifested as significant HR acceleration(16,39–41) similar to that seen in our study.
In conclusion, our data and those from the literature identify HR acceleration induced by BMs as a fundamental reflex response that develops similarly in utero and ex utero in premature and full-term infants.
Abbreviations
- ANS:
-
autonomic nervous system
- AS:
-
active, REM sleep
- BM:
-
spontaneous body movements
- GA:
-
gestational age in weeks
- HR:
-
heart rate
- QS quiet:
-
NREM sleep
- %RRM:
-
percentage of RR modification induced by body movement.
- wGA:
-
weeks gestational age
- EEG:
-
electroencephalographic
- IS:
-
indeterminate sleep
- ANOVA:
-
analysis of variance
References
Mitchell JH 1990 Neural control of circulation during exercise. Med Sci Sports Exerc 22: 141–154.
Haskel WL, Yee MC, Evans A, Irby PJ 1993 Simultaneous measurements of heart rate and body motion to quantitate physical activity. Med Sci Sports Exerc 25: 109–115.
Gasser HS, Meek WJ 1914 A study of the mechanisms by which muscular exercise produces acceleration of the heart. J Physiol (Lond) 34: 48–71.
Brunia CHM 1984 Facilitation and inhibition in the motor system: an interrelationship with cardiac deceleration. In: Coles MGH, Jennings JR, Stern JA (eds) Psychophysiological Perspectives. Van Nostrand Reinhold Company, New York, 3–19.
Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG 1985 Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337.
Willamson JW, Nobrega ACL, Winchester PA, Zim S, Mitchell JH 1995 Instantaneous heart rate increase with dynamic exercise: central command and muscle-heart reflex contributions. J Appl Physiol 78: 1273–1279.
Baust W, Bohnert B 1969 The regulation of heart rate during sleep. Exp Brain Res 7: 169–180.
Fagraeus L, Linnarsson D 1976 Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol 40: 679–682.
Porges SW 1995 Orienting in defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology 32: 301–318.
Townsend RE, Johnson LC, Naitoh P, Muzet AG 1975 Heart rate preceding motility in sleep. Psychophysiology 12: 217–219.
Muzet A, Michel C 1977 Heart rate preceding short activation phases in sleep. Waking Sleep 1: 175–179.
Alihanka J 1982 Sleep movements and associated autonomic nervous activities in young male adults. Acta Physiol Scand 511: suppl 1–85.
Smirne S, Ferini-Strambi L, Zucconi M, Pinto P, Franceschi M 1990 Cardiac autonomic dysfunction during sleep in some neurological diseases. Neurophysiol Clin 20: 131–136.
Ferini-Strambi L, Franceschi M, Pinto P, Zucconi M, Smirne S 1992 Respiration and heart rate variability during sleep in untreated Parkinson patients. Gerontology 38: 92–98.
Erkinjunti M, Kero P 1985 Heart rate response to body movements in healthy and neurologically damaged infants during sleep. Early Hum Dev 12: 31–37.
Di Pietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TRB 1996 Development of fetal movement-fetal heart rate coupling from 20 wk through term. Early Hum Dev 44: 139–151.
Siassi B, Hodgman JE, Cabal L, Hon EH 1979 Cardiac and respiratory activity in relation to gestation and sleep states in newborn infants. Pediatr Res 13: 1163–1166.
Curzi-Dascalova L 1992 Physiological correlates of sleep development in premature and full-term newborns. Neurophysiol Clin 22: 151–166.
Clairambault J, Curzi-Dascalova L, Kauffmann F, Medigue C, Leffler C 1992 Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum Dev 28: 169–183.
Goujard J, Kaminski M, Rumeau-Rouquette C 1973 Moyenne pondérale et âge gestationnel en relation avec quelques caractéristiques maternelles. Arch Franc Ped 30: 341–362.
Blondel B, Kaminski M, Kabir M, Dargent-Paré C, Tuppin Ph, Bréart G 1991 Mortalité et morbidité périnatales en France. In: Tourner M (ed) Collège National Gynécologique et Obstétrique Français. Vigout, Paris, 175–213.
Curzi-Dascalova L, Mirmiran M 1996 Manual of Methods of Recording and Analyzing Sleep-Wakefulness States in Preterm and Full-Term Infants. Editions INSERM, Paris
Apgar J, James LS 1962 Further observation of the newborn scoring system. Am J Dis Child 104: 419–428.
Peirano P, Curzi-Dascalova L, Vicente G 1986 Influence of states of sleep and age on body motility in normal premature and full-term neonates. Neuropediatrics 17: 186–190.
Spassov L, Curzi-Dascalova L, Clairambault J, Kauffmann F, Eiselt M, Medigue C, Peirano P 1994 Heart rate and heart rate variability in small-for-gestational-age newborns. Pediatr Res 35: 500–505.
Kauffman F, Amorim R, Curzi-Dascalova L 1996 Analysis of heart rate during movements in sleeping premature and full-term newborns. In: Conference Proceedings 1996 IEEE Engineering in Medicine and Biology 18th Annual International Conference, Amsterdam 484–485.
Curzi-Dascalova L, Christova E, Peirano P, Singh BB, Gaultier CI, Vicente G 1989 Relationships between respiratory pauses and heart rate during sleep in normal premature and full-term newborns. J Dev Physiol 11: 323–330.
Eiselt M, Curzi-Dascalova L, Leffler Ch, Christova E. 1992 Sigh-related heart rate changes during sleep in premature and full-term newborns. Neuropediatrics 23: 286–291
Marlot D, Duron B 1979 Postnatal maturation of phrenic, vagus and intercostal nerves in kitten. Biol Neonate 36: 264–272.
Gootman PM 1983 Neural regulation of cardio-vascular function in the perinatal period. In: Gootman N, Gootman PM (eds) Perinatal Cardiovascular Function. Marcel Dekker Inc, New York, 265–327.
VanRavenswaaij-Arts CMA, Hopman JCW, Kollée LAA, van Amen JPL, Stoelinga GBA, van Geijn HP 1991 Influence of heart rate variability in spontaneously breathing preterm infants. Early Hum Dev 27: 187–205.
Curzi-Dascalova L, Spassov L, Eiselt M, Peirano P, Kauffmann F, Clairambault J, Medigue C 1994 Development of cardio-respiratory control and sleep in newborns. In: Cosmi AV, Renzo GC (eds) Current Progress in Perinatal Medicine. London, The Parthenon Publishing Group Ltd, 303–308.
Lagercrantz H, Edwards D, Henderson-Smart D, Hertzberg T, Jeffery H 1990 Autonomic reflexes in preterm infants. Acta Paediatr Scand 79: 721–728.
Stothers JK, Warner RM 1978 Oxygen consumption and neonatal sleep states. J Physiol (Lond) 278: 435–440.
Bach V, Bouferrache B, Kremp O, Maingourd Y, Libert JP 1994 Regulation of sleep and body temperature in response to exposure to cool and warm environments in neonates. Pediatrics 93: 789–796.
Speidel BD 1978 Adverse effect of routine procedures on preterm infants. Lancet 1: 864–866.
Long JG, Philip AGS, Lucey JF 1980 Excessive handling as a cause of hypoxemia. Pediatrics 65: 203–207.
Abu-Osba YX, Brouillette RT, Wilson SL, Thach BT 1982 Breathing pattern and transcutaneous oxygen tension during motor activity in preterm infants. Am Rev Respir Dis 125: 382–387.
Navot D, Yaffe H, Sadovsky E 1984 The ratio of fetal heart rate accelerations to fetal movements according to gestational age. Am J Obstet Gynecol 149: 92–94.
Dawes GS, Visser GHA, Goodman JDS, Levine DH 1981 Numerical analysis of the human heart rate: modulation by breathing and movement. Am J Obstet Gynecol 140: 535–544.
Johnson TRB, Besinger RE, Thomas RL, Strobino DM, Niebyl JR 1992 Quantitative and qualitative relationships between fetal heart rate acceleration and fetal movement. J Matern Fetal Med 1: 251–253.
Author information
Authors and Affiliations
Additional information
Supported by Conselho National de Desenvolvimento Cientifico et Technolocico/Brazil (R.H.C.d.A.).
Department of Mathematics, Université de Caen, FR-14032, Caen, France
Department of Pediatrics, Facultade de Medicina, Universidade Federal de Minas Gerais, Belo Horisonte, Brazil
Rights and permissions
About this article
Cite this article
Curzi-Dascalova, L., Kauffmann, F., Gaultier, C. et al. Heart Rate Modifications Related to Spontaneous Body Movements in Sleeping Premature and Full-Term Newborns. Pediatr Res 45, 515–518 (1999). https://doi.org/10.1203/00006450-199904010-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199904010-00010
This article is cited by
-
Fewer spontaneous arousals during prone sleep in preterm infants at 1 and 3 months corrected age
Journal of Perinatology (2006)
-
How to score arousals in preterm infants?
Wiener Klinische Wochenschrift (2003)