Abstract
A novel copper-binding protein was identified in Indian childhood cirrhosis (ICC) of the liver supernatant (1000 × g), purified to apparent homogeneity, and characterized. Purified major copper-binding protein (MCuBP) is solely responsible for binding about 35% of the total supernatant copper. Elution profile of ICC liver supernatant on Sephadex G-75 column chromatography showed three peaks. About 60% of the total supernatant copper was resolved in peak II, whereas zinc content was insignificant in this peak. But peak II was almost missing in a gel elution profile of control liver supernatant. The control group included cases of various liver diseases viz. neonatal hepatitis, septicemia, and mixed nodular cirrhosis. Copper-binding proteins of peak II further purified on ion-exchange chromatography and elution profile showed that peak II was a MCuBP with high copper-binding capacity (10 g atoms/mol of native protein). SDS-PAGE of this protein also revealed the existence of a single band with molecular mass of about 50 kD. UV spectra of MCuBP showed the maximal absorbance at 254 nm. Unlike the classical metallothionein, the amino acid composition of MCuBP revealed the presence of aromatic amino acids and higher content of glutamic acid and aspartic acid followed by glycine and serine. The ratio (0.3) of basic amino acids to acidic amino acids strongly indicates that it is an acidic protein. The cysteine content in this protein was insignificant, which further corroborates the possibility that the acidic amino acids might be prominent candidates for binding copper. Thus, the 50-kD MCuBP apparently makes a major contribution to the total copper-binding activity in ICC liver cytosol and may play a significant role in hepatic intracellular copper accumulation.
Similar content being viewed by others
Main
ICC is a lethal copper storage disease (1–3). Portmann and his colleagues described the presence of striking orcein-positive deposits of copper-binding protein in liver biopsy specimens from ICC patients (1,2). Subsequent studies showed that the copper content of the livers of ICC patients was several times higher than in any disease so far reported, such as Wilson's disease and primary biliary cirrhosis (1,2,4,5). Two disorders of copper transport, Menkes disease and Wilson disease, have been well studied from clinical and biologic aspects (6). The recent cloning of the defective genes for these diseases has begun to shed light on the specific proteins involved in cellular transport (7,8).
Copper is an indispensable micronutrient, functioning as a cofactor in a variety of enzymes including cytochrome c oxidase, superoxide dismutase, tyrosinase, dopamine 8-hydroxylase, and lysyl oxidase (9,10). Regulation of intracellular copper activity is crucially important for cell viability. Excess copper can be lethal, which acts predominantly through the formation of highly reactive hydroxide radicals, which can damage cell membrane, DNA, mitochondria, and proteins (11–13). Indeed, copper homeostasis requires appropriate mechanisms for copper absorption, cellular transport, incorporation into protein, storage, and excretion. In mammalian systems various proteins have been recognized for these functions (14), albumin for copper transport in the blood, ceruloplasmin as a possible copper donor to tissue and enzymes (15), and MT for intracellular copper storage (16). MTs are low molecular mass (6 kD), cysteine-rich metal-binding proteins found in eukaryotes, which are involved in copper and zinc homeostasis and in metal detoxification (17). Sternleib (17) has reported that at least 80% of the copper of the normal mammalian liver is dispersed in the cytosol where it is bound predominantly to proteins of 10 and 30 kD (16). The low molecular weight protein corresponds to MT, which is a primary acceptor and main distributor of copper internalized by mammalian hepatocytes. Current understanding of hepatic copper metabolism in the ICC liver with reference to proteins responsible for the accumulation and intracellular processing of copper remains unclear.
In the present study, we have identified and physicochemically characterized from the liver of ICC patients a novel cytosolic, MCuBP of molecular mass of approximately 50 kD, which has a very high tenacity to bind copper. This novel protein contains a very high proportion of acidic amino acids, i.e. aspartate and glutamate. Thus, this protein is different from the classical metal-binding protein (MT) in reference to molecular weight amino acid composition and capacity to bind copper. The high levels of MCuBP along with elevated copper levels in the ICC liver strongly suggest that this protein may be either the primary defect in the ICC disease or a secondary response due to excess copper accumulation.
METHODS
Materials. Sephadex G-75 and DEAE-Sephadex A-50 were purchased from Pharmacia, Uppsala, Sweden. 64Cu (specific activity 20 GBq/g of copper) and 110mAg (specific activity 10 GBq/g of silver) were procured from Bhabha Atomic Research Center, Trombay, Bombay, India. All other chemicals used were of analytical grade.
Collection of samples. Postmortem specimens of the liver of children in whom the diagnosis of ICC disease had been established by clinical, biochemical, and histopathologic criteria, i.e. 1) presence of Mallory's hyaline, 2) presence of pericellular fibrosis, 3) presence of neutrophilic exudate, 4) ineffective nodulation, and 5) diffuse excess of copper-binding protein as demonstrated by orcein stains (3), were obtained immediately after death from Nehru Hospital, Postgraduate Institute of Medical Education and Research, Chandigarh. The patient had not received D-penicillamine therapy during admission. Postmortem liver tissue from control individuals with liver diseases, such as neonatal hepatitis, septicemia, active mixed nodular cirrhosis, were taken for the study. All subjects in both groups were male and less than 5 years of age. Specimens were stored at -70°C until used.
Estimation of metals (copper and zinc) and MT in human liver. Copper and zinc levels in tissues were estimated by the wet digestion and dry weight methods as previously described (18). For wet digestion, the sample was digested in 5 mL of digestion mixture (HNO3:HC1O4, 5:1) in a digester (Model-Buchi 445, Switzerland) to dryness, and the residue was reconstituted in 10 mM nitric acid. For the dry weight method, the sample was dried at 90°C for 48 h in an incubator, and dried tissue weight was recorded. Further, the dried sample was processed as in wet digestion methods. Copper and zinc were monitored on an atomic absorption spectrometer (Perkin Elmer-4000) at 327.4 and 201.1 nm, respectively. The instrument was calibrated using the Sigma copper and zinc standards. Bovine liver standard reference material SRM-1577 obtained from the National Bureau of Standards (Washington, DC) was used for quality control.
The frozen liver sample (0.5 g) was homogenized in an ice bath in 4 volumes of 0.25 M sucrose solution with a Potter-Elvehjem homogenizer. The homogenates were centrifuged at 10,000 × g for 20 min in a Sorvall centrifuge (Ivan Sorvall Inc., Norwalk, CT). The supernatant was assayed for MT by the silver-hemoglobin method (19). Aliquots of 0.2 mL of the supernatant were adjusted to a sample volume of 1.2 mL with 0.5 M glycine buffer solution, pH 8.5. The sample solutions were mixed with 1 mL of radioactive silver nitrate solution (110mAg+, 20 ppm solution). The excess 110mAg+ was removed by three consecutive hemolysate, heat, and centrifugation treatments. The 110mAg bound to MT was determined by measuring the radioactivity in autogamma scintillation counter (1282 Compugamma, Universal Gamma Counter).
Preparation of liver cytosols. The unfrozen liver was rinsed with normal saline, cut into small pieces, and homogenized in 4 volumes of 10 mM Tris-HCl buffer, PMSF (40 µg/mL), pH 8.0, using a Potter-Elvehjem homogenizer with a Teflon pestle for 20 strokes. After filtering through four layers of cheese cloth, the homogenate was centrifuged at 105,000 × g for 1 h in Beckman Ultracentrifuge RC-2B at 4°C to obtain cytosol.
Purification of MCuBP. All steps in purification procedure described below were carried out at 4°C. The protease inhibitor PMSF was added to each buffer to give a final concentration of 100 µM.
Gel filtration chromatography of proteins. Copper-binding proteins were purified from liver cytosol using Sephadex G-75 (1.5 × 100 cm) preequilibrated with 10 mM Tris-HCl buffer, pH 8.0. Ten milliters of supernatants from different preparations containing about 170-180 mg of protein were charged on the column and were eluted with the same buffer at a flow rate of 10 mL/h. Fractions of 2.5 mL were collected and analyzed for copper and zinc. The absorbances were measured at 280 and 254 nm on a Hitachi U-3200 Spectrophotometer. Total protein content was measured by the method of Lowry et al. (20).
The copper content in peak II was about 67% of the total copper in charged liver cytosol. Partially purified peak fractions (II) were pooled and concentrated by ultrafiltration, which was carried out in an ultrafiltration unit (Amicon cell using ultrafiltration membrane UM2, Amicon Inc.) under nitrogen at a pressure of 55 psi.
DEAE-Sephadex A-50 ion-exchange chromatography of protein. Two milliliters of a concentrated pool of partially purified copper-binding protein obtained from a Sephadex G-75 column was charged on an ion-exchange column (2 × 10 cm) of DEAE-Sephadex A-50 preequilibrated with 10 mM Tris-HCl buffer, pH 8.0. After charging, the column was washed with 1 volume of 10 mM Tris-HCl, pH 8.0, before developing the column with a linear gradient of 10 mM to 1 M Tris-HCl, pH 8.0. Three-milliliter fractions were collected at a flow rate of 12 mL/h and monitored for copper and absorbance at 280 nm. The chloride gradient of ion exchange chromatography was monitored by the method of Schales and Schales (21).
Fast protein liquid chromatography. A Mono Q HR 5/5 (Pharmacia) column (5 × 50 mm) was used to separate protein and to check the purity of protein from partially purified copper-binding protein obtained on Sephadex G-75 and ion-exchange chromatography, respectively. The Mono Q column was equilibrated with the starting buffer (10 mM Tris-HCl, pH 8.0, containing PMSF (40 µg/mL). Samples (1 mL) containing 1 mg of protein and 5 µCi of 64Cu(II) were incubated for 10 min at 37°C and passed through a filter (0.2 µm, Millex GV, Millipore Corp., Bedford, MA). A 500-µl sample was applied to the Mono Q column through a loop. After eluting the protein, which did not bind to the column in the starting buffer (10 mM Tris-HCl, pH 8.0), a linear salt gradient (0-1 M NaCl) in 10 mM Tris-HCl, pH 8.0, was applied. Fractions of 0.5 mL were collected. The 64Cu radioactivity in each fraction was counted in autogamma scintillation counter and corrected for decay.
SDS-PAGE. The homogeneity of purified protein was tested by SDS-PAGE according to the method of Laemmli (22) under reducing conditions. The stacking gel was 6% and the separating gel was 12%. The gel thickness was 0.75 mm. The gels were stained with Coomassie Brilliant Blue as described by Johnson et al. (23).
Molecular weight estimation. The molecular mass of purified ICC liver cytosolic copper-binding protein was determined by SDS-PAGE using a mixture of fructose-6-P kinase (84 kD), BSA (67 kD), pyruvate kinase (58 kD), lactate dehydrogenase (38 kD), and triose isomerase (26 kD) as molecular mass reference proteins purchased from the Sigma Chemical Co. (St. Louis, MO).
Spectral studies of MCuBP. Ultraviolet spectra of purified MCuBP were recorded at pH 8.0 in the presence of 0.5 M D-penicillamine/0.5 M histidine on a double beam Hitachi U-3200 spectrophotometer. UV spectra were also recorded at pH 2.0 after the addition of HCl in the cuvette.
Amino acid analysis. Purified MCuBP was hydrolyzed in vacuo at 110°C for 24 h with twice distilled HCl. Half-cysteine was determined as cysteic acid after performic acid oxidation (24). Hydrolysate samples were neutralized with triethylamine and derivatized with phenylisothiocyanate. Amino acid analysis was done on a Waters PICO tag amino acid analyzer calibrated with standard amino acids.
Statistical analysis. Results are expressed as means ± SE and compared by an unpaired t test with significance defined as p < 0.05.
RESULTS
Relationship of MT levels with copper and zinc concentrations in liver. Table 1 illustrates that the concentration of copper in the ICC liver was approximately 29-fold higher than that found in control liver, expressed either as per gram wet of tissue or per gram of dry tissue. On the other hand, hepatic zinc levels were similar in both groups. There was considerable individual to individual variation in ICC hepatic copper, whereas hepatic zinc levels were far more uniform. These results indicate a specific massive accumulation of copper in ICC livers. There was no significant change in hepatic MT levels and the Zn/MT ratio in both groups. However, the ratio of Cu/MT was about 27 times higher in ICC liver in comparison with control liver. Mean hepatic copper content in the control group was 1.5-fold higher than in normal liver (10 µg/g wet weight). Therefore, it is a possibility that protein other than MT would be the prominent component responsible to bind intracellular copper in ICC hepatocytes.
Purification of MCuBP. The copper content in ICC liver supernatant (1000 × g) was about 9-fold higher than in the control liver supernatant. Moreover, the hepatocytes in ICC liver showed orcein-stained granules, providing evidence of copper-binding proteins. Purification of MCuBP was carried out as described under "Methods." As summarized in Table 2, MCuBP was purified about 12-fold over the copper binding activity in ICC liver cytosol. The yield of this protein was 4% of the total cytosolic protein.
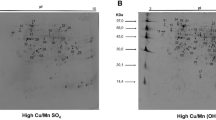
Gel filtration chromatography. Representative gel filtration elution profiles on Sephadex G-75 from control and ICC liver cytosolic proteins are shown in Figure 1, A and B. Three copper-binding peaks were detected. These are labeled viz. peaks I, II, and III, which correspond to different molecular masses [>75 kD, ≈50 kD, and low molecular mass protein, i.e. MT (10 kD) respectively], as estimated by comparison with standard markers (data not shown). These peak fractions differ in copper, zinc, and protein content (Table 3). The average yield of the copper and zinc applied to the Sephadex G-75 was ≈80%. In the control elution profile, peak I fractions contained ≈50% copper, peak II ≈14% copper, and 28% copper in MT fractions (peak III), whereas ICC liver cytosol showed a typical elution pattern on Sephadex G-75. A substantial amount of copper and protein was detected in peak II fractions, which were eluted after the high molecular weight proteins fractions (peak I). It is noteworthy here that copper content (130 µg) in peak II fractions contributes ≈59% of the total cytosolic copper, whereas the zinc content in this peak was insignificant. Peak I and peak III fractions contained ≈22 and 6.8% copper, respectively, although the copper content in these peak fractions was significantly higher when compared with the copper content of the corresponding peaks in the control liver cytosolic elution profile. Representative patterns of zinc in control and ICC liver cytosol elution profiles were very similar in reference to their residual zinc content as well as percent zinc of total cytosolic zinc. Thus, higher copper load in ICC liver did not perturb the zinc levels in these peak fractions, which is indicative of eventual stabilization of zinc binding capacities of these protein fractions. It should be noted that ceruloplasmin activity was observed only in peak I fractions in control and ICC liver cytosol profiles. Interestingly, no Cu/Zn superoxide dismutase activity was detected in peak II fractions of the ICC liver cytosol profile. Therefore, peak II fractions most likely contain copper-binding proteins other than ceruloplasmin and Cu/Zn superoxide dismutase.
Fractionation of liver cytosolic copper-binding proteins by gel permeation chromatography. The column (1.5 × 100 cm) of Sephadex G-75 was equilibrated with 10 mM Tris-HCl buffer, pH 8.0. About 180 mg of protein (10 mL of supernatant) were applied. Proteins were eluted with 10 mM Tris-HCl, pH 8.0, buffer containing 100 µM PMSF: Fractions (2.5 mL) were collected at a flow rate of 10 mL/h. Copper and zinc were monitored on an atomic absorption spectrophotometer in all the fractions, directly (A), normal cytosol (B), and ICC cytosol (C) ICC heat-treated cytosol (80°C for 10 min). ▴–▴ depicts copper µg/mL; ×–×, zinc µg/mL; ∧^, absorbance at 280 nm; and •–•, absorbance at 254 nm.
Peak II of the control liver cytosolic elution profile is very minute in size, whereas this peak in ICC liver cytosolic elution profile is drastically elevated. For the confirmation of peak II in the control liver cytosol, 64Cu-labeled cytosol was fractionated on Sephadex G-75 (Fig. 2). A significantly higher ratio of 64Cu to OD at 280 was observed in peak II fractions. Although the protein content in the peak II fractions was substantially low, 64Cu counts in the other two peaks was comparable to the copper content as shown in Figure 1A. Hence it confirms the presence of copper-binding proteins in peak II whose quantity is extremely low in control liver cytosol. To determine the heat stability of copper-binding proteins in peak II of ICC liver cytosolic elution profiles, the ICC liver cytosol was heated at 80°C for 10 min in a water bath and then centrifuged at 10,000 × g for 30 min. Soluble material was subjected to Sephadex G-75 column. The elution profile of soluble supernatant showed one major peak III, which represents MT (Fig. 1C). This indicates that most of the high molecular weight proteins of peak I and copper-binding proteins of peak II were denatured. The findings suggest that copper-binding proteins in peak II are heat-labile and MTs (peak III) are heat-stable. Moreover, copper and zinc contents of MTs (peak III) were comparable to that of the untreated peak III (Fig. 1B).
Representative gel filtration profile of 64Cu in control liver cytosol. 64Cu, 10 µCi, was added to the 10- mL supernatant (≈180 mg of protein) and incubated at 37°C for 10 min, then passed through a 0.2-µm membrane filter. The sample was applied to a Sephadex G-75 column (1.5 ×–× 100 cm), equilibrated with 10 mM Tris-HCl buffer, pH 8.0. The proteins were eluted with 10 mM Tris-HCl, pH 8.0, containing 100 µM PMSF. Fractions, 2.5 mL, were collected at a flow rate of 10 mL/h. 64Cu radioactivity was counted in all fractions. ∧–^ depicts absorbance at 280 nm; •–•, absorbance at 254 nm; and ▴–▴, 64Cu cpm/mL fraction.
Anion exchange chromatography. Anion exchange chromatography disclosed that peak II proteins were resolved into two distinct peaks viz. peak I and peak II (Fig. 3). Peak II protein was MCuBP, which contributes about 60% of the total copper charged on the anion exchange column (Table 4). Zinc content in this peak was insignificant; peak I fractions had 22% of charged copper, which was eluted before applying the gradient. Peak II fractions were collected, pooled, and dialyzed overnight against 10 mM Tris-HCl, pH 8.0, buffer. Pooled fractions were then concentrated using a UM-2 membrane. The concentrated proteins were stored at -70°C after freezing in liquid nitrogen at a concentration of 1 mg/mL in buffer containing 10 mM Tris-HCl, 100 µM PMSF, pH 8.0, for further studies.
DEAE-Sephadex A-50 chromatography profile of copper binding protein from ICC liver. Peak II fractions (31-46) from Sephadex G-75 chromatography were pooled and concentrated. A 1-mL concentrated sample containing ≈6 mg of protein was applied to DEAE-Sephadex A-50 column (2 × 10 cm). The column was equilibrated with 10 mM Tris-HCl, pH 8.0, buffer. Proteins were eluted with a linear gradient of sodium chloride (0-1 M) at a flow rate of 12 mL/h. Fractions of 3 mL were collected, and copper was estimated directly by aspiration of the sample on atomic absorption spectrophotometer •–• depicts absorbance at 280 nm, ▴–▴, the Cu µg/mL fraction, and (—) the chloride gradient.
Resolution pattern of ICC cytosolic protein obtained on gel and ion-exchange columns by FPLC using a Mono Q column. Peak II proteins obtained on gel chromatography when labeled with 64Cu and charged on a Mono Q column resolved into two distinct peaks on FPLC (Fig. 4). Rechromatography on a Mono Q column FPLC increased resolution, yet the elution profile was similar to DEAE-Sephadex A-50. There were two copper-binding proteins in which peak II was the MCuBP and had 60% counts/min of charged 64Cu. Peak II proteins obtained on DEAE-Sephadex A-50 were also subjected to a Mono Q column. Only a sharp single peak was eluted in the same position with the same sodium chloride gradient strength (Fig. 4). Most of the 64Cu was associated with MCuBP.
Rechromatography of ICC hepatic copper-binding proteins on FPLC. 64Cu (5 µCi) was added separately to concentrated copper-binding proteins (1 mg/mL) obtained from Sephadex G-75 (A) and DEAE-Sephadex A-50 (B) columns. Both the samples were fractionated separately on a Mono Q column (5 × 50 mm), preequilibrated with 10 mM Tris-HCl, pH 8.0. Proteins were eluted using a linear gradient formed between 10 M Tris-HCl buffer, pH 8.0, and the same buffer containing 1 M NaCl at a flow rate of 1 mL/min with 0.5-mL fractions. Radioactivity of 64Cu (×–×) was counted in all the fractions which were collected according to absorbance at 280 nm (——) displayed on chart recorder.
Characterization of MCuBP. The copper content in the native MCuBP was found to be 14.50 µg of copper/mg of protein (Table 2), which is equivalent to 10 g atoms of copper/mol of MCuBP.
Molecular mass and subunit composition. Final preparation of MCuBP obtained on DEAE-A-50 gave a single band of protein after electrophoresis of the denatured protein on a 12% polyacrylamide gel (Fig. 5). The molecular mass of the MCuBP was found to be 50 kD. Analysis of the subunit structure of the purified MCuBP by SDS-PAGE revealed only a single band with a molecular mass of 50 kD.
PAGE of MCuBP. Aliquots of about 10 µg of purified MCuBP were applied to gels under nondenaturating and denaturating conditions. Protein was detected by Coomassie Brilliant Blue staining. Standard proteins were used fructose-6-phosphate kinase (84 kD), BSA (67 kD), pyruvate kinase (58 kD), lactate dehydrogenase (36 kD), and triose isomerase (26 kD). (a) Electrophoresis of the standard marker proteins on 12% polyacrylamide gel, (b) electrophoresis of the purified MCuBP on a 12% polyacrylamide gel under nondenaturating condition, and (c) electrophoresis of the purified MCuBP on a 12% polyacrylamide gel under denaturating conditions. The direction of migration is from top to bottom.
Spectral studies. The UV spectral analysis of purified MCuBP is shown in Figure 6. There was an absorption maximum showing the shoulder at 254 nm, which is a peculiar characteristics of metal protein interactions. Either in the presence of chelating agents such as D-penicillamine or in the form of histidine, there was a disappearance of a shoulder at 254 nm, suggesting the dissociation of copper from the protein. However, lowering of the pH (<2.0) of the protein solution could not abolish the absorption maximum, reflecting high affinity of copper to the protein.
Amino acid composition. The amino acid analysis revealed that MCuBP is an acidic protein that contained high levels (residues/1000 residues) of glutamic acid (182) and aspartic acid (100). These two amino acids contribute 28.2% of the total amino acid composition. Basic amino acids such as lysine (54), arginine (37), and histidine (30) were also present in this protein. Unlike the classical MT, aromatic amino acids such as phenylalanine (36) and tyrosine (10) were also present in MCuBP. Surprisingly, no cysteine molecule, a prominent amino acid in classical MT, was found in this protein.
DISCUSSION
Massive accumulation of copper in the ICC liver was accompanied by orcein-stained hepatocyte granules, as reported earlier (3,4), which provides evidence of copper-binding proteins in ICC hepatocytes. A novel MCuBP (50 kD) was identified and purified from ICC liver cytosol. It could be a predisposing factor for massive accumulation of copper, and it was present in high amounts in ICC liver. MCuBP is responsible for binding 35% of total cytosolic copper and an insignificant amount of zinc. Interestingly, extremely low levels of MCuBP were observed in control liver cytosol.
The only molecule for which a clear role in intracellular metal metabolism has been ascribed is MT (14). The involvement of MT in hepatic copper metabolism is quite complex. When Cu2+ and Zn2+ accumulate intracellularly, the reactive ions can be sequestered in a chemically innocuous form by binding to newly synthesized apo-MT. This mechanism is believed to account for the accumulation of a large amount of Cu-MT in tissues and cells of organisms affected with inherited disorders of copper metabolism (17,25–27). Measurement of hepatic copper during the course of primary biliary cirrhosis has demonstrated that the copper level increases with the duration of the disease process and reflects the severity of cholestasis (28). It has been suggested that the increased hepatic copper plays a role in the development of cirrhosis. It is noteworthy in primary biliary cirrhosis that copper is associated with the protein that has been identified as MT. In Wilson's disease, where there is also a massive accumulation of copper in liver which leads to liver cirrhosis, the presence of copper-saturated hepatic MT and its elevated levels in liver have been reported (35). However, despite the high copper content in ICC liver (580 µg/g of wet tissue), there was no significant change in MT contents as well as levels of Cu2+ and Zn2+ bound to MT in ICC compared with control liver. Feeding of high or low levels of copper diet had little or no effect on MT accumulation (29). From their results, it was apparent that the liver copper binding isoform of MT increased above basal level only when the liver copper concentration had increased to above 600 µg of copper/g of wet tissue. Normal induction of MT synthesis has also been observed in response to copper and zinc in fibroblasts from children with ICC (30). Thus, our results on MT accumulation in ICC liver are totally consistent with the reported one. Therefore, it is inferred that an etiology other than impaired MT production could be a primary defect in this disorder.
UV spectral analysis of the purified MCuBP also strongly indicates the presence of a copper-protein complex because it had absorbance maxima at 254 nm (Fig. 6). These findings are in accordance with previous reports (31,32). Surprisingly, the shoulder at 254 nm did not disappear by lowering the pH to 2.0, indicating that copper is bound to protein in Cu(I) form which may account for stronger binding to protein considering the fact that Cu(II) usually forms planar compounds, whereas Cu(I) forms tetrahedral compounds (33,34). In the present study, the shoulder at 254 nm disappeared in the presence of chelating agents such as D-penicillamine and histidine as reported previously (34,35). Thus, D-pencillamine found its place as an effective chelator for decoppering in ICC patients (36).
MCuBP isolated from the ICC liver differs with respect to amino acid composition from that of classical MT (Table 5). MCuBP also differs from MT in Molecular mass, heat stability, absence of cysteine, presence of aromatic amino acids, and high tenacity to bind copper. In contrast to MT, virtually no zinc is present in MCuBP. The ratio (0.3) [(Σ Lys + Σ Arg)/(Σ Glu + Σ Asp)] in MCuBP indicates that it is an acidic protein. Sulfhydryl groups play a major role in binding copper in MT. But, it appears that due to lack of cysteine residues in MCuBP, the alternative binding moieties to copper are glutamate, aspartate, serine, and histidine, which are the most prominent amino acids in this protein. Free carboxyl groups of glutamate and aspartate, the hydroxyl group of serine, and the imidazole group of histidine may provide secondary affinity binding sites to metals (37,38). Notwithstanding the defect in ICC, in the present study it appears that the levels of 50-kD MCuBP observed in ICC disease in somehow involved in the pathophysiology of this disease. The 50-kD MCuBP appears to have a high affinity for copper, and its levels are affected in lethal ICC disease. These facts are consistent with a central role for the MCuBP component in intracellular copper metabolism. Recently, a copper-binding protein of similar molecular weight was well correlated with the hepatic copper level in brindled mice (39). This is a significant finding, because to date MT is the only other known intracellular protein which was thought to play role in copper metabolism. It may be concluded that a novel protein MCuBP, apart from MT, potentially plays a vital role in cellular copper metabolism. Abnormal elevated levels of MCuBP might be the basic defect in ICC disease. Further studies on cloning and functional characterization of MCuBP are in progress.
Abbreviations
- ICC:
-
Indian childhood cirrhosis
- MCuBP:
-
major copper-binding protein
- MT:
-
metallothionein
- PMSF:
-
phenylmethylsulfonyl fluoride
- FPLC:
-
fast protein liquid chromatography
References
Portmann B, Tanner MS, Mowat AP, Williams R 1978 Orcein positive liver deposits in Indian childhood cirrhosis. Lancet 1: 1338–1340
Tanner MS, Portmann B, Mowat AP, Williams R, Pandit AN, Mills CF, Bremmer I 1979 Increased hepatic copper in Indian childhood cirrhosis. Lancet 1: 1203–1205
Bhagwat AG, Walia BNS 1980 Indian childhood cirrhosis: commentary. Indian J Pediatr 48: 433–437
Adamson M, Reiner B, Olson JL, Goodman Z, Plotnick L, Bernardini I, Gahl WA 1992 Indian childhood cirrhosis in an American child. Gastroenterology 102: 1771–1777
Popper H, Goldfisher S, Sternlieb I, Nayak NC, Madhavan TV 1979 Cytoplasmic copper and its toxic effects. Studies in Indian childhood cirrhosis. Lancet 1: 1205–1208
Cox DW 1995 Genes of the copper pathway. Am J Hum Genet 56: 828–834
Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J 1993 Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper transporting ATPase. Nat Genet 3: 7–13.
Petrukhin KE, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC 1994 Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Hum Mol Genet 3: 1647–1656
Turnlund JR 1994 Copper. In: Shils ME, Olson JA, Shike M (eds) Modern Nutrition in Health and Disease, 8th Ed. Lea & Febiger, Philadelphia, pp 231–241.
Olivares M, Uauy R 1996 ) Copper as an essential nutrient. Am J Clin Nutr 63: 791S–796S
Sagripanti JL, Krammer KH 1989 Site specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. J Biol Chem 264: 1729–1739
Aruoma OI, Halliwell B, Gajewski E, Dizetaroglu M 1991 Copper ion dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J 273: 601–604
Prasad R, Kaur G, Nath R, Walia BNS 1996 Molecular basis of pathophysiology of Indian childhood cirrhosis: role of nuclear copper accumulation in liver. Mol Cell Biochem 156: 25–30
Sarkar B 1981 Transport of copper. In: Sigel, H (ed) Metal Ions in Biological Systems. Marcel Dekker, New York, pp 223–261.
Linder MC, Hazegh-Azam M 1996 Copper biochemistry and molecular biology. Am J Clin Nutr 63: 797S–811S
Hammer DH 1986 Metallothionein. Annu Rev Biochem 55: 913–951
Sternleib T 1987 Hepatic lysosomal copper-thionein. Exper Suppl 52: 647–653
Vig PJS, Paliwal VK Nath R 1991 A comparative study of direct current plasma, atomic emission spectrometry and atomic absorption spectrophotometer for biological monitoring of trace metals. In: Dhillon HK, Hu MH (eds) Biological Monitoring of Exposure to Chemicals and Metals. John Wiley & Sons, New York, pp 163–171.
Scheuhammer AM, Cherian MG 1986 Quantification of metallothioneins by a silver saturation method. Toxicol Appl Pharmacol 82: 417–425
Lowry OH, Rosebrough NJ, Farr AL, Randall RL 1951 Protein measurement with Folin phenol reagent. J Biol Chem 193: 265–775
Schales O, Schales SS 1941 A simple and accurate method for the determination of chloride in biological fluids. J Biol Chem 140: 879–884
Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature 227: 680–685
Johnson GF, Morell AG, Stockert RJ, Sternlieb I 1981 Hepatic lysosomal copper protein in dogs with an inherited copper toxicosis. Hepatology 1: 243–248
Hirs CHW 1967 Hirs CHW (ed) Methods in Enzymology, Vol II. Academic Press, New York, pp 197–199.
Nartey NO, Frei JV, Cherian MG 1987 Hepatic copper and metallothionein distribution in Wilson's disease (hepatolenticular degeneration). Lab Invest 57: 397–401
Packman S, Palmiter RD, Karin M, O'Toole C 1987 Metallothionein messenger RNA regulation in the mottled mouse and Menkes kinky hair syndrome. J Clin Invest 79: 1338–1342
Herzberg NH, Wolterman RA, Berg GJ, Barth PG, Bolhius PA 1990 Metallothionein in Menke's disease: induction in cultured muscle cells. J Neurol Sci 100: 50–56
Janssens AR, Bosman PT, Ruiter DJ, Van den Hammer CJA 1984 Immunohistochemical demonstration of the cytoplasmic copper associated proteins in the liver in the primary biliary cirrhosis: its identification as metallothinein. Liver 4: 139–147
Bremner I, Mehra RK, Morrison JN, Wood AM 1986 On the occurrence of metalothionein I in the liver and its secretion into blood, bile and urine. Biochem J 235: 735–739
Hahn SH, Tanner MS, Danks DM, Gahl WA 1995 Normal metallothionein synthesis in fibroblasts obtained from children with Indian childhood cirrhosis or copper associated childhood cirrhosis. Biochem Mol Med 54: 142–155
Kaur G, Nath R, Gupta GS 1993 Purification and characterization of low molecular weight metal binding from human testes. J Trace Elem Exp Med 6: 1–13
Nielson KB, Atkin CL, Winge DR 1985 Yeast metallothionein. Sequence and metal binding properties. J Biol Chem 260: 14464–14470
Schuberg J 1964 Copper and peroxides. In: Radiobiology and Medicine. Charles C Thomas, Springfield, IL, pp 17–19.
Kagi JHR 1991 Overview of metallothionein. Methods Enzymol 205: 613–615
Li TY, Kraker AJ, Shaw CF, Petering DH 1980 Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc Natl Acad Sci USA 77: 6334–6338
Bhusnurmath SR, Walia BNS, Singh S, Prakash D, Radotra BD, Nath R 1991 Sequential histopathological alterations in Indian childhood cirrhosis treated with D-pencillamine. Hum Pathol 22: 653–658
Jacobson KB, Turner JE 1980 Interaction of cadmium with certain other metal ions with proteins and nucleic acids. Toxicology 16: 1–37
Antholine WE, Petering DH, Pickart L 1989 ESR studies of the interaction of copper II histidine and Ehrlich cells. J Inorg Biochem 35: 215–224
Palida FA, Ettinger MJ 1991 Indentification of proteins involved in intracellular copper metabolism: low levels of a ∼48 kD copper binding protein in the brindled mouse model of Menkes disease. J Biol Chem 266: 4586–4592
Acknowledgements
The authors thank Prof. B. N. Datta, Head, Department of Pathology, PGIMER, Chandigarh, for histopathologic studies and Prof. G. P. Talwar, N.I.I., New Delhi for analysis of amino acid.
Author information
Authors and Affiliations
Additional information
Supported by PGIMER, Chandigarh, and Department of Biotechnology, New Delhi, India.
Rights and permissions
About this article
Cite this article
Prasad, R., Kaur, G., Mond, R. et al. Identification of a Novel Copper-Binding Protein from Indian Childhood Cirrhosis of the Liver: Purification and Physicochemical Characterization. Pediatr Res 44, 673–681 (1998). https://doi.org/10.1203/00006450-199811000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199811000-00009