Abstract
Recent studies in immature fetal animals demonstrated only a slight or variable increase in the cerebral glycolytic rate during moderate isocapnic hypoxemia. However, the methods used in those studies did not allow for detection of small differences or of regional redistributions of the cerebral glycolytic rate. Hence, a global increase or a regional redistribution of the cerebral glycolytic rate during hypoxemia accompanied by a severe increase in tissue lactate concentration in a few brain areas may have been overlooked in these studies. Because these pathophysiologic mechanisms seem to considerably exacerbate neuronal cell damage due to hypoxic/ischemic insults, we were keen to clarify this point. We, therefore, applied the 2-deoxyglucose method to fetal guinea pigs in utero and measured total and regional cerebral glucose utilization in fetuses of this species at 0.75 of gestation during maternal isocapnic hypoxemia. At 0.75 of gestation guinea pig dams were chronically catheterized. Control groups were exposed to room air, whereas study groups were exposed to a hypoxic atmosphere (10% oxygen, 2% carbon dioxide, and 88% nitrogen). To measure total and regional cerebral glucose utilization during normoxemia and isocapnic hypoxemia, we injected i.v. 100μCi of 2-[3H]deoxyglucose into the dams. Total and regional cerebral glucose utilization were determined from the steady-state clearance of 2-deoxyglucose between the maternal arterial plasma and the fetal brain, the glucose concentration in the maternal arterial plasma, and the “lumped constant.” During isocapnic hypoxemia, total fetal cerebral glucose utilization was not significantly higher than that previously measured during normoxemia (8 ± 0.8 versus 8 ± 1.0 μmol/100 g/min). Furthermore, no redistribution of cerebral glucose utilization could be detected. We conclude that moderate isocapnic hypoxemia in the immature fetal brain does not lead to any significant increase or redistribution of glucose utilization or to any major lactate accumulation. This may be related to the low cerebral metabolic demands of brain tissue at this stage of development. Whether this is the main reason for the known resistance of the immature fetal brain toward ischemic neuronal cell damage remains to be established.
Similar content being viewed by others
Main
Hypoxic-ischemic cerebral damage is an important contributor to perinatal mortality and morbidity including long-term neurologic sequelae, especially in preterm fetuses(1). As shown in previous studies lactate accumulation caused by acceleration of anaerobic glycolysis during hypoxic/ischemic insults seem to aggravate neuronal cell damage(2, 3). At present there are only a few studies available, in which cerebral glucose utilization and lactate accumulation were measured in immature fetuses during isocapnic hypoxemia. Using the Fick method, Asano et al.(4) determined cerebral glucose utilization in immature fetal sheep at 0.75 of gestation during 8 h of maternal isocapnic hypoxemia. Over this period in time no change in the cerebral glycolytic rate and oxygen consumption was detected, but there was a considerable lactate efflux from the brain. Furthermore, studies on fetal guinea pigs at a comparable neurologic maturity(5) demonstrated a slight increase in lactate concentration of the cerebral cortex after 1 h of maternal isocapnic hypoxemia(6). However, because the Fick method is insensitive to small net fluxes, when blood flow is high, and the fetal guinea pig has a high capacity to transport lactate across the blood-brain barrier(7), a severe increase in anaerobic glycolysis in immature fetal brain during isocapnic hypoxemia cannot be excluded by these studies. In addition, there may be also a regional redistribution of the glycolytic rate within the fetal brain during hypoxemia accompanied by a severe increase in tissue lactate concentration in various areas. Because such a severe accumulation of lactate seems to aggravate neuronal cell damage after hypoxic/ischemic insults(2, 3), we wished to examine these important questions. We, therefore, applied the 2-deoxyglucose method(8) to the fetal guinea pig in utero(9) and measured total and regional cerebral glucose utilization in fetuses of this species at 0.75 of gestation during maternal isocapnic hypoxemia. A brief account of this study has been published elsewhere(10).
METHODS
We studied 83 fetuses from 32 albino guinea pigs at 50 ± 1 d of gestation (term is at 68 d). The dams were anesthetized by subcutaneous injection of 0.05 mL of a 2% solution of xylazine (Rompun, Bayer AG, Leverkusen, Germany) and 50 mg/kg ketamine hydrochloride (Ketanest, Parke-Davis, München, Germany). Polyvinyl catheters were inserted into the maternal carotid artery and jugular vein. The animals were allowed to recover from anesthesia and surgery for at least 2 d. The dams were studied in a cylindrical Perspex box(6). Control groups were exposed to room air, and study groups were subjected to a hypoxic atmosphere(10% oxygen, 2% carbon dioxide, and 88% nitrogen)(6). Maternal arterial blood was sampled at 10-min intervals to determine blood gases, blood pH, and plasma glucose concentrations (278 blood gas system, Ciba Corning, Frankfurt, Germany; Glucose Analyzer 2, Beckman, Fullerton, CA). Acute hypoxia has been shown to stimulate cerebral glucose metabolism initially, but to lead to the establishment of a new steady-state within a few minutes of continued exposure to hypoxia(11–13). We, therefore, allowed 15 min for the dams to adapt to the hypoxic environment before starting tracer experiments.
Using the 2-deoxyglucose method in the fetal guinea pig total and regional cerebral glucose utilization can be determined from the steady-state clearance of 2-deoxyglucose between the maternal arterial plasma and the fetal brain, the glucose concentration in the maternal arterial plasma, and the“lumped constant”(9). In this modification of the original method the lumped constant is defined as the ratio between the steady-state clearance of 2-deoxyglucose and glucose between the maternal arterial plasma and the fetal brain. We recently determined the lumped constant using this model during normoxemic conditions(9). However, because hypoxemia may change the lumped constant, by lowering maternal arterial Po2, which could alterate glucose concentrations in the various maternal and fetal distribution pools, we decided to reinvestigate the lumped constant. Hence, in the present study the lumped constant was determined from the steady-state distribution volumes of 3-O-methylglucose (Ve') and glucose(Vf) between the maternal arterial plasma and the fetal brain, and the ratio of the phosphorylation coefficients of 2-deoxyglucose(k5*) and glucose(k5) in the fetal brain (ϕ)(9, 14, 15). The steady-state clearance of 2-deoxyglucose (K*) and the steady-state distribution volumes of 3-O-methylglucose (Ve') and glucose(Vf) between the maternal arterial plasma and the fetal brain were measured using the same theoretical and experimental procedures recently described for normoxemia(9).
The steady-state clearance of 2-deoxyglucose (K*) and the steady-state distribution volume of 3-O-methyglucose(Ve') between the maternal arterial plasma and the fetal brain were measured by i.v. bolus injection of 100 μCi of 2-[3H]deoxyglucose and 20 μCi of 3-o-[14C]methylglucose into the dams (25 fetuses from 9 dams). Samples from maternal arterial plasma were obtained between 10 and 75 min after injection. The steady-state clearance of 2-deoxyglucose(K*) and the steady-state distribution volume of 3-O-methylglucose (Ve') were assessed according toEquations 1 and 2: where V* is the distribution volume of 2-deoxyglucose in the fetal brain taken as the concentration of 2-deoxyglucose in the fetal brain(M*(T)) versus the concentration of the 2-deoxyglucose in the maternal plasma at the end of the experiment(Ca*(T)). Θ* represents the normalized plasma integral taken as the time integral of 2-deoxyglucose concentration in the maternal plasma∫oTCa(t)dt versus the arterial concentration of 2-deoxyglucose at the end of the experiment (Ca*(T)). Vo is a correction for nonextracted tracer in the vascular bed .Vg* is the “dynamic” volume of the distribution of nonmetabolized radiolabeled 2-deoxyglucose in the fetal brain.eqn. 2 where Me'(T) is the concentration of 3-O-methylglucose in the fetal brain and Ce'(T) the concentration 3-O-methylglucose in the maternal plasma at the end of the experiment.
A second set of experiments (13 fetuses from 5 dams) was designed to measure the total cerebral glucose consumption (Jglc) of the guinea pig fetuses 45 min after i.v. injection of 100 μCi of 2-[3H]deoxyglucose into the dams according toEquation 3: In this group of animals the time-dependent distribution volume of 2-deoxyglucose in the fetal brain(V*), the normalized time-integral of 2-deoxyglucose(Θ*) in maternal plasma and the steady-state glucose concentration in the maternal plasma (Ca) were determined in each experiment, whereas the value of the term (Vg* -Vo) was derived from the first set of experiments.
The lumped constant was calculated from the steady-state distribution volumes of 3-O-methylglucose (Ve') and glucose(Vf) between the maternal arterial plasma and the fetal brain, and the ratio of the phosphorylation coefficient of 2-deoxyglucose to that of glucose in the fetal brain ϕ(15). This procedure assumes that the coefficient for the backward transport of 2-deoxyglucose from the fetal brain to the maternal arterial plasma(k2'*) is much larger than the phosphorylation coefficient of 2-deoxyglucose in the fetal brain(k5*): eqn. 4 Inasmuch as the ratio between the phosphorylation coefficients (ϕ) should be confined to very narrow limits, even if the velocity of the individual processes,i.e. forward and backward transport of hexoses across the blood-brain barrier as well as phosphorylation of hexoses in the fetal brain, varies in different pathologic states(16, 17), we have taken the value recently reported for fetal guinea pigs during normoxic conditions (ϕ = 0.28)(9). To determine the steady-state distribution volume of glucose [Vf =Me(T)/Ca(T)] between the maternal arterial plasma and the fetal brain, the maternal arterial plasma glucose concentration was measured in 6 other dams. In the corresponding fetuses (n = 13) the glucose concentrations in the cerebral cortex(Me) were determined using spectrophotometry after immersing the fetuses in liquid nitrogen(18, 19).
In a final set of experiments regional cerebral glucose utilization was studied during normoxemia (17 fetuses from 6 dams) and isocapnic hypoxemia (15 fetuses from 6 dams). Forty-five minutes after injection of 100 μCi of 2-[3H]deoxyglucose the dams were decapitated, and the fetal brains were removed as in the former groups. The brains were then rapidly dissected into the following regions: frontal lobe, temporal lobe, occipital lobe, caudate nucleus, hippocampus, thalamus, midbrain, cerebellum, and brainstem. Regional cerebral glucose utilization was calculated according toEquation 3, where the value for(Vg* - Vo) was derived from the first set of experiments. Radioactivity in fetal brain and maternal plasma samples in each experimental set-up was measured by liquid scintillation spectrometry(9).
The experimental protocols were approved by the appropriate institutional review committee and met the guidelines of the responsible governmental agency.
Chemicals. Solutions of 2-deoxy-D-[1-3H]glucose and 3-O-methyl-D-[U-14C]glucose were obtained from Amersham Buchler, Braunschweig, Germany, with specific activities of 370 and 1.85 GBq/mmol, respectively.
Statistics. The results are given as means ± SD. Linear regression analysis was used for statistical evaluation of curves. The significance of differences between groups was established by Mann Whitney test or by analysis of variance and Scheffé post hoc test, where necessary.
RESULTS
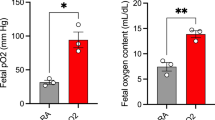
After 15 min of hypoxia, maternal Po2 fell by 50%, whereas Pco2 and pH remained constant (Table 1). From then onward, there was no significant change in maternal blood gas variables or plasma glucose concentrations until the end of the experiments. In comparison with our normoxemic experiments reported previously(9), maternal isocapnic hypoxemia resulted in a decrease in fetal mixed arteriovenous Po2 of about 40% without any change in fetal Pco2 and pH (Table 1).
Between 10 and 75 min after the injection of 2-deoxyglucose into the dams, the regression between the normalized integral in the maternal arterial plasma and the distribution volume in the fetal brain approximated linearity(V* = 0.38 + 0.0066 Θ*; r = 0.93,p < 0.001) (Equation 1), indicating that the clearance of 2-deoxyglucose from the maternal arterial plasma to the fetal brain was in a steady-state (Fig. 1).
The steady-state distribution volume of glucose (Vf) was 0.36 ± 0.08 mL/g. Between 10 and 75 min after injection of 3-O-methylglucose there was no change in the distribution volume of this glucose analog, confirming the secular equilibrium between the maternal plasma and fetal brain glucose pool, i.e. constant ratio of specific activities. The values were therefore averaged (0.72 ± 0.04 mL/g). The lumped constant determined from the steady-state distribution volumes of 3-O-methylglucose (Ve') and glucose(Vf) was 0.56 (Equation 4).
As shown in Table 2, total cerebral glucose utilization of the fetal brain during hypoxemia was 8 ± 0.8 μmol/100 g/min. This was identical to the value previously measured for normoxemia using the lumped constant determined from the steady-state distribution volumes of 3-O-methylglucose (Ve') and glucose(Vf) between the maternal arterial plasma and the fetal brain, and the ratio of the phosphorylation coefficients of 2-deoxyglucose and glucose in the fetal brain (ϕ) [see Berger et al.(9), Table 3 and 4, Equations 31 and 32, Λ = 0.583].
In the experiments in which we measured fetal regional glucose utilization during isocapnic hypoxemia, the only physiologic variables of the study group that differed from those of the control group were maternal and fetal arterial Po2 (Table 3). Furthermore, no significant change was observed in either maternal blood gases or plasma glucose concentration after tracer injection until the end of the experiments.
Because no differences in regional cerebral glucose utilization were found between the left and right hemispheres, paired brain structures were pooled. During isocapnic hypoxemia, fetal regional cerebral glucose utilization remained unchanged in all brain structures studied (Table 4).
DISCUSSION
This study shows that in immature fetuses there is no significant increase in total cerebral glucose utilization during maternal isocapnic hypoxemia. Furthermore, we detected no redistribution of regional cerebral glucose utilization within the brain. This observation raises the question of whether anaerobic glycolytic metabolism is accelerated under these experimental conditions. Depending on the degree of cerebral oxygen consumption there are two possibilities to consider. First, if cerebral oxygen consumption is well maintained during maternal isocapnic hypoxemia as shown in immature sheep fetuses(20), a considerable acceleration in anaerobic glycolysis and lactate accumulation(6) is very unlikely in the present study. Second, if cerebral oxygen consumption is reduced during moderate maternal hypoxemia, anaerobic glycolysis may significantly increase. This is not necessarily accompanied by cerebral lactate accumulation, because transport capacity for lactate across the blood-brain barrier is high in fetal guinea pigs(7). However, a reduction of fetal cerebral oxidative metabolism has not yet been shown for moderate maternal hypoxemia. Thus, this hypothesis is unlikely to be true. At any rate, severe lactate accumulation (>10 μmol/g) with subsequent neuronal cell damage in the fetal brain can be largely excluded under these experimental conditions. The results of the present study and previous reports(4, 6) are quite compatible with an insignificant acceleration of the cerebral glycolytic rate in the immature guinea pig followed by a minimal global increase in tissue lactate concentration of about 3 μmol/g after 1 h of isocapnic hypoxemia.
Because we were unable to detect major increases in cerebral glycolysis during hypoxemia, the question arises whether the redox state of the brain remained stable due to compensatory increases in cerebral blood flow. Alternatively, one might speculate that the immature brain lacks the normal metabolic ATP/ADP feedback control on glycolytic enzymes. There are studies to support both views. First, during isocapnic maternal hypoxemia immature sheep fetuses are able to maintain total cerebral oxygen consumption by increasing cerebral blood flow and oxygen extraction fraction(4, 20). In addition, there is no change in tissue concentrations of high energy phosphates in the cerebral cortex under these experimental conditions as shown for fetal guinea pigs at a comparable neurologic maturity(6). In the present study an acceleration of the anaerobic glycolytic rate due to an alteration of cerebral redox state with subsequent ATP/ADP feedback control on glycolytic enzymes is, therefore, very unlikely. Second, as shown in mature sheep fetuses and adult animals, there is an acceleration of the cerebral glycolytic rate under similar experimental conditions(21–23) followed by an increase in tissue lactate concentration to about 10 μmol/g without any change in ATP/ADP ratio(22, 23). Whether there are still alterations of the ATP/ADP ratio in the various microenvironments of the neurons under these conditions, or whether there are additional factors beside changes in ATP/ADP ratio that are involved in the control of the glycolytic rate during isocapnic hypoxemia, is at present unclear. However, it may be conceivable that there are ontogenetic differences in these hypothesized mechanisms causing differences in the acceleration of cerebral glycolytic rate. This may attenuate the known acceleration of the cerebral anaerobic glycolytic rate in the immature fetus during isocapnic hypoxemia and, hence, may protect its brain from neuronal cell damage.
One might speculate that an increase in the cerebral glycolytic rate caused by a breakdown of glycogen stores was not detected in this study, because the deoxyglucose method does not measure changes in glucose utilization resulting from glycogenolysis. However, there are two arguments against this speculation. First, as shown in immature fetal sheep, cerebral glycogen concentrations are well maintained under these experimental conditions(24). Second, the cerebral glucose concentration does not change in immature fetal guinea pigs during isocapnic hypoxemia(6), and the cerebral glycolytic rate is in a steady-state, because the slope of the Θ versus V curve in Figure 1 does not change significantly. In the present study, therefore, an increase in cerebral glucose utilization due to glycogenolysis, that of course would not have been measured by the deoxyglucose method, is very unlikely.
There is one study in the literature showing a significant increase in cerebral lactate efflux in immature fetal sheep with no change in glucose and oxygen uptake during prolonged isocapnic hypoxemia(4). These results are difficult to interpret. The authors speculate that the observed lactate efflux may result from substrate sources other than glucose. However, at present there are no experimental data confirming this hypothesis.
In the present study a slight, but insignificant, decrease in glucose utilization was observed in the brainstem during isocapnic hypoxemia(Table 4). This contrasts with the preferential increase in cerebral blood flow to the brainstem observed by Gleason et al.(20) in immature sheep fetuses during maternal isocapnic hypoxemia. In that study oxygen delivery to the brainstem was found to be maintained, whereas that to the cerebellum and to the cerebral hemispheres declined during isocapnic hypoxemia. This may help the immature fetus to preferentially protect the brainstem, because an acceleration in anaerobic glycolysis is not necessary for energy production given sufficient oxygen supply. This view is supported by studies in newborn puppies, in which an uncoupling of glucose utilization and blood flow in the brainstem was demonstrated during hypoxia(13, 25).
The calculation of the lumped constant was based onEquation 4, where ϕ is derived from experiments performed under normoxic conditions(9). This approximation is compatible with the original method provided two conditions are fulfilled(14, 15): first, that the variableϕ does not change, and second, that the coefficient for the backward transport of 2-deoxyglucose from the fetal brain to the maternal arterial plasma is much larger than the phosphorylation coefficient of 2-deoxyglucose in the fetal brain. These conditions are met, because 1) irrespective of the experimental setup ϕ is known to be confined to narrow limits(16, 17) and 2) according to our previous study(9) the coefficient for the backward transport of 2-deoxyglucose from the fetal brain to the maternal plasma is much larger than the phosphorylation coefficient of 2-deoxyglucose in the fetal brain.




Abbreviations
- C a :
-
glucose concentration in the maternal arterial plasma
- Ca*(T):
-
concentration of 2-deoxyglucose in the maternal arterial plasma at the end of the experiment
- Ce'(T):
-
concentration of 3-O-methylglucose in the maternal arterial plasma at the end of the experiment
- ϕ:
-
ratio of the phosphorylation coefficients of 2-deoxyglucose to that of glucose in the fetal brain
- K * :
-
steady-state clearance of 2-deoxyglucose between the maternal arterial plasma and the fetal brain
- k2'*:
-
coefficient of the backward transport of 2-deoxyglucose from the fetal brain to the maternal arterial plasma
- k 5 * :
-
phosphorylation coefficient of 2-deoxyglucose in the fetal brain
- k 5 :
-
phosphorylation coefficient of glucose in the fetal brain
- J glc :
-
glucose utilization in the fetal brain
- Λ:
-
lumped constant
- M*(T):
-
concentration of 2-deoxyglucose in the fetal brain at the end of the experiment
- Me(T):
-
concentration of glucose in the fetal brain at the end of the experiment
- Me'(T):
-
concentration of 3-O-methylglucose in the fetal brain at the end of the experiment
- Θ*:
-
normalized integral of 2-deoxyglucose concentration in the maternal arterial plasma
- V o :
-
correction term for nonextracted tracer in the fetal vascular bed
- V * :
-
time-dependent distribution volume of 2-deoxyglucose in the fetal brain
- Ve':
-
steady-state distribution volume of 3-O-methylglucose in the fetal brain
- V f :
-
steady-state distribution volume of glucose in the fetal brain
- Vg*:
-
dynamic distribution volume of nonmetabolized 2-deoxyglucose in the fetal brain
- ∫0TCa(t)dt:
-
time integral of 2-deoxyglucose concentration in the maternal arterial plasma
References
Volpe JJ 1987 Neurology of the Newborn. WB Saunders, Philadelphia.
Kalimo H, Rehncrona S, Söderfeldt B, Olsson Y, Siesjö BK 1981 Brain lactic acidosis and ischemic cell damage. 2. Histopathology. J Cereb Blood Flow Metab 1: 313–327.
Myers RE 1977 Experimental models of perinatal brain damage: relevance to human pathology. In: Gluck L (ed) Intrauterine Asphyxia and the Developing Fetal Brain. Year Book, New York, pp 37–97.
Asano H, Homan J, Carmicheal L, Korkola S, Richardson B 1994 Cerebral metabolism during sustained hypoxemia in preterm fetal sheep. Am J Obstet Gynecol 170: 939–944.
Dobbing J, Sands J 1979 Comparative aspects of the brain growth spurt. Early Hum Dev 3: 79–83.
Berger R, Jensen A, Krieglstein J, Steigelmann J-P 1993 Cerebral energy metabolism in fetal guinea pigs during moderate maternal hypoxemia at 0.75 of gestation. J Dev Physiol 19: 193–196.
Bissonnette JM, Hohimer AR, Chao CR 1991 Unidirectional transport of glucose and lactate into brain of fetal sheep and guinea pig. Exp Physiol 76: 515–523.
Sokoloff L, Reivich M, Kennedy C, Des Rousiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M 1977 The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28: 897–916.
Berger R, Gjedde A, Heck J, Müller E, Krieglstein J, Jensen A 1994 Extension of the 2-deoxyglucose method to the fetus in utero: theory and normal values for the cerebral glucose consumption in fetal guinea pigs. J Neurochem 63: 271–279.
Berger R, Gjedde A, Hargarter L, Hargarter S, Krieglstein J, Jensen A 1993 Fetal cerebral glucose consumption during maternal hypoxemia. J Cereb Blood Flow Metab 13:S415.
Borgström L, Norberg K, Siesjö BK 1976 Glucose consumption in rat cerebral cortex in normoxia, hypoxia and hypercapnia. Acta Physiol Scand 96: 569–574.
Duffy TE, Nelson SR, Lowry OH 1972 Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem 19: 959–977.
Duffy TE, Cavazutti M, Cruz NF, Sokoloff L 1982 Local cerebral glucose metabolism in newborn dogs: effects of hypoxia and halothane anesthesia. Ann Neurol 11: 233–246.
Gjedde A, Diemer NH 1983 Autoradiographic determination of regional brain glucose content. J Cereb Blood Flow Metab 3: 303–310.
Gjedde A 1982 Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: a re-examination. Brain Res Rev 4: 237–274.
Crane PD, Pardridge WM, Braun LD, Oldendorf WH 1983 Kinetics of transport and phosphorylation of 2-fluoro-2-deoxy-D-glucose in rat brain. J Neurochem 40: 160–167.
Dyve S, Gjedde A 1991 Glucose metabolism of fetal rat brain in utero, measured with labeled deoxyglucose. Acta Neurol Scand 83: 14–19.
Berger R, Jensen A, Krieglstein J, Steigelmann J-P 1991 Effects of acute asphyxia on brain energy metabolism in fetal guinea pigs near term. J Dev Physiol 16: 9–11.
Bergmeyer HU 1970 Methoden der enzymatischen Analyse. Verlag Chemie, Wienheim.
Gleason CA, Hamm C, Jones D 1990 Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol 258:H1064–H1069.
Hohimer AR, Chao CR, Bissonnette JM 1991 The effect of combined hypoxemia and cephalic hypotension on fetal cerebral blood flow and metabolism. J Cereb Blood Flow Metab 11: 99–105.
Siesjö BK, Nilsson L 1971 The influence of arterial hypoxemia upon labile phosphates and upon extracellular and intracellular lactate and pyruvate concentrations in the rat brain. Scand J Clin Lab Invest 27: 83–96.
Beck T, Krieglstein J 1987 Cerebral circulation, metabolism, and blood-brain barrier of rats in hypocapnic hypoxia. Am J Physiol 252:H504–H512.
Wagner KR, Ting P, Westfall MV, Yamaguchi S, Bacher JD, Myers RE 1986 Brain metabolic correlates of hypoxia-ischemic cerebral necrosis in mid-gestational sheep fetuses: significance of hypotension. J Cereb Blood Flow Metab 6: 425–434.
Cavazutti M, Duffy TE 1982 Regulation of local cerebral blood flow in normal and hypoxic newborn dogs. Ann Neurol 11: 247–257.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berger, R., Gjedde, A., Hargarter, L. et al. Regional Cerebral Glucose Utilization in Immature Fetal Guinea Pigs during Maternal Isocapnic Hypoxemia. Pediatr Res 42, 311–316 (1997). https://doi.org/10.1203/00006450-199709000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199709000-00011