Abstract
The goal of these experiments was to determine whether the perturbation of ischemia-reperfusion has an age-dependent effect on subsequent endothelial cell production of nitric oxide. Three-and 35-d-old swine in the experimental group were exposed to 1-h partial ischemia (90% flow reduction) and 2-h reperfusion in vivo by creation and then removal of a mesenteric artery coarctation. Control subjects underwent exposure of the mesenteric artery only. After reperfusion, gut vascular resistance had increased 44± 6% in 3-d-old, but had decreased 41 ± 4% in 35-d-old subjects. At the completion of the in vivo portion of the protocol mesenteric artery was removed, and nitric oxide production was estimated in vitro, by measuring cGMP production by vessel segments or by measuring relaxation of phenylephrine-precontracted rings, both after stimulation of nitric oxide production by substance P or the calcium ionophore A23187. Compared with control, mesenteric artery segments from 3-d-old subjects demonstrated reductions in basal, substance P-stimulated (10-8 M) and A23187-stimulated (10-7 M) cGMP accumulation of 50 ± 7%, 66± 6% and 78 ± 7%. Mesenteric artery segments from 35-d-old subjects demonstrated increases in basal, substance P-stimulated, or A23187-stimulated cGMP accumulations of 114 ± 14%, 92 ± 8%, or 78 ± 9%. Compared with control, I/R rings from 3-d-old subjects demonstrated reductions in substance P-induced (10-8 M) or A23187-induced (10-7 M) relaxations of 56 ± 7% or 30 ± 7%. In contrast, 35-d-old ischemia-reperfusion rings demonstrated increases in substance P- or A23187-induced relaxation of 36 ± 8% or 98 ± 11%. It is concluded that ischemia-reperfusion has an age-dependent effect on endothelial production of NO within in vitro postnatal mesenteric artery and that these changes mirror the effects of ischemia-reperfusion on gut vascular resistance in vivo.
Similar content being viewed by others
Main
Twenty-five years after publication of Lloyd's(1) report proposing a link between asphyxia-induced intestinal ischemia and gastrointestinal perforation in newborn infants, the role of circulatory events in the development of NEC remains an enigma.
Lloyd's proposal, later dubbed the “diving reflex” theory, suggested that redirection of cardiac output occurred during birth asphyxia, causing gut ischemia and eventual gut necrosis. Subsequent laboratory studies by Touloukian et al.(2) and Alward et al.(3) seemed to confirm the feasibility of Lloyd's theory, firmly entrenching a putative relationship between ischemia and NEC in the lore of neonatal medicine. Several clinical studies later challenged this concept. Most importantly, it became clear that the majority of infants afflicted with NEC never experienced asphyxia, or any other substantial circulatory event requisite to Lloyd's theory(4, 5). Final dismissal of ischemia as a participant in the etiology of NEC has never occurred, however, because histopathology clearly suggests the existence of an hypoxic-ischemic event at some time during the disease process(6, 7).
Is there another means to link the circulation to the pathogenesis of NEC? Virtually all proposed associations view the intestinal circulation as an innocent bystander; for example, circumstances believed to induce gut ischemia, such as asphyxia(1), umbilical artery cannulation(8), or polycythemia(9), originate at a site distal to the gut vasculature. An alternative approach is to implicate intestinal vascular pathology as the principal mediator of relevant gut ischemia. For example, disruption of the vascular endothelium might lead to aberrant hemodynamics, inasmuch as these cells transduce chemical and mechanical stimuli to vasoactive signals which determine the contractile state of adjacent smooth muscle, and so regulate local blood flow(10, 11). Studies in the coronary circulation have confirmed the feasibility of this scenario. Transient occlusion of a coronary artery damages endothelial cells distal to the site of blockage; consequently, myocardial hemodynamics remain impaired despite removal of the initial occlusion because endothelial cell production of vasoactive factors, especially relaxing factors, is attenuated(12–14). Thus, a brief ischemic event initiates endothelial cell pathology within the distal microvasculature, which in turn leads to sustained ischemia within small areas of myocardium and eventual tissue damage(13). Two lines of evidence suggest that a similar process might occur in newborn intestine. First, endothelium-derived relaxing factors, especially NO, participate in setting basal vascular tone in newborn intestine, as evidenced by the rise in gut vascular resistance after administration of arginine analogs which curtail NO production(15). This circumstance suggests that endothelial cell damage might have a similar effect on gut hemodynamics. Second, sustained intestinal vasoconstriction is noted in 3-d-old but not 35-d-old subjects after a brief period of ischemia(16), a perturbation known to disrupt endothelial cell function in other circulations(12–14).
The purpose of these experiments was to determine the effect of ischemia-reperfusion on endothelial cell function in mesenteric artery from 3- and 35-d-old swine. The perturbation of ischemia-reperfusion was chosen as the means to induce endothelial cell damage, inasmuch as it is reproducible and easy to achieve, and also because of its potential clinical relevance in the pathogenesis on NEC. NO production was chosen as the marker for endothelial cell function because it is the principal endothelium-derived relaxing factor(11) and because of its established role in regulation of postnatal gut hemodynamics(15). Two standard means were used to estimate NO production in vitro: relaxation of precontracted vessel rings in response to agents known to stimulate NO production(17), and accumulation of cGMP, the second messenger engaged by NO within vascular smooth muscle(18).
METHODS
Animals. Postnatal swine were chosen as the experimental subject because of their physiologic similarity to the human newborn(19). Subjects were obtained from local swine farms on the day before study and were fasted for 12 h before surgery. Two age groups were studied. Subjects in the 3-d-old group had an age range of 2-4 d and weight range of 1.9-2.4 kg. Subjects in the 35-d-old group had an age range of 32-37 d and weight range of 6.8-7.6 kg. In several instances littermates were studied on sequential days. All procedures were carried out under anesthesia with pentobarbital sodium (35 mg/kg to induce, 10 mg/kg maintenance) and euthanasia was achieved by administration of Uthol (1 mL/kg). The experimental protocols were approved and the work monitored by the Institutional Animal Care and Use Committee of the Children's Hospital Research Foundation.
Subjects from each age group were divided into two subgroups. Experimental subjects were exposed to ischemia-reperfusion in vivo, whereas control subjects underwent an identical surgical preparation, excluding only mesenteric artery ligation. The mesenteric artery removed from each control subject was divided and used in both the cGMP and ring tension assays; eight 3- and eight 35-d-old subjects were used as controls. The mesenteric artery removed from the experimental subjects was used in either the cGMP or the ring tension assays; thus, a total of sixteen 3-d-old and sixteen 35-d-old subjects were used, eight each for the cGMP and ring tension assay.
Induction of ischemia-reperfusion in vivo. The goal of the experimental method was to induce partial, not total, ischemia, inasmuch as partial ischemia would be more likely to occur under natural circumstances and is thus a more clinically relevant perturbation. To this end, a method was developed to reduce intestinal blood flow to ≈10% of baseline by inducing a transient coarctation in the main mesenteric artery trunk just distal to the aorta. Subjects were anesthetized, intubated, and ventilated to maintain normal blood gas tensions, and vascular catheters were placed in a carotid artery and jugular vein. The mesenteric artery and aorta were exposed in the retroperitoneal space by flank incision, and a heavy silk tie was placed around the mesenteric artery. Thereafter, the viscera were exposed via a midline abdominal incision, heparin was administered (500 U/kg), the mesenteric vein trunk was cannulated, and the abdominal wound was closed. This catheter was directed to a collection flask. Blood was continuously pumped from the flask back to the subject at a rate equal to venous outflow, so that euvolemia of the study subject was maintained. An electromagnetic flowmeter was placed in-line with the mesenteric vein circuit to measure intestinal flow rate. Placement of the flowmeter on the venous side of the intestine was used to avoid trauma to the arterial vasculature, which was later used forin vitro analyses. The site of flowmeter placement does not affect the accuracy of flow measurement in the swine gut inasmuch as its circulation is derived from a single mesenteric artery, rather than from multiple sources as is the case in other species. Once steady-state conditions were attained, as evidenced by stability of intestinal flow rate and systemic blood pressure, subjects in the experimental group had a mesenteric artery coarctation induced by placing a short cylindrical piece of solid plastic between the tie and vessel, tightening the tie, and thereafter removing the plastic cylinder. This action left a coarctation whose external diameter approximated that of the cylinder (3 mm in 3-d-old and 6 mm in 35-d-old subjects). The coarctation was left in place for 1 h; thereafter, the tie was removed, and reperfusion was allowed to proceed for 2 h. During this time mesenteric venous flow rate and pressure and arterial pressure were measured continuously. Intestinal vascular resistance was calculated as the ratio between aortic-mesenteric vein pressures and mesenteric vein flow rate. Arterial blood samples were obtained throughout the protocol for blood gas and blood oxygen content analysis. Subjects in the control group underwent an identical surgical preparation, except that the mesenteric artery tie was not tightened. These control subjects were observed for 3 h after preparation, a time similar to that of the ischemia-reperfusion protocol.
Vessel preparation. Upon completion of the in vivo phase of the study the mesenteric artery was removed en bloc from its aortic root to its distal ileal branches and immediately immersed in iced Krebs buffer; thereafter, the subject was euthanized. A segment of artery between the first and last jejunal branches was dissected free of the mesentery and cut into 4-mm rings, with care being taken not to touch the luminal surface. All dissections were carried out on a tray filled with Krebs buffer, situated on ice so that the buffer and tissue remained very cold.
Ring tension experiments. Method. Rings were mounted between two stainless steel stirrups placed within water-jacketed 20-mL glass wells. One stirrup was fixed to the bottom of the well, whereas the second was tethered to a force transducer (Grass FT03) to facilitate continuous measurement of isometric tension. The well was filled with Krebs buffer of the following composition (in mM): NaCl 118.1, KCl 4.8 CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, glucose 11.1, disodium EDTA 0.026. When aerated with 95% O2-5% CO2 at 38°C, the buffer pH was 7.40. This buffer was maintained at 38°C by running warm water through the jacketing system surrounding the wells. Rings were progressively stretched over 1-2 h to to optimal point on their length-tension curve as determined by noting maximal contractile response to 20 mM KCl. Indomethacin (10-5 M) was added to all buffer to prevent eicosanoind production. Endothelial integrity was confirmed in all rings by noting ≥50% relaxation in response to acetylcholine (10-7 M), an endothelium-dependent vasodilator(20).
Protocol. In this protocol, NO production was estimated by measuring relaxation of isometric tension in phenylephrine-precontracted mesenteric artery rings in response to agents known to exert their effect by activation of the NO-cGMP axis. Three agents were studied. Substance P binds to an endothelial cell membrane receptor; this receptor-ligand complex then increases movement of Ca2+ into the cell, an action which activates NO synthase(20). The Ca2+ ionophore A23187 directly increases Ca2+ flux into the endothelial cell without prior receptor binding and so provides a direct stimulus for NO synthase activation(20). Sodium nitroprusside is a nitrovasodilator which directly activates vascular smooth muscle soluble guanylate cyclase, entirely bypassing endothelial cell nitric oxide synthase; in this context, it serves as a marker for vascular smooth muscle function(20). Rings were precontracted to ≈75% of their maximal tension with phenylephrine (1-5 × 10-6 M, as necessary). Thereafter, dose-response curves were generated for substance P (10-11-10-8 M), the calcium ionophore A23187 (10-9-10-6 M), and sodium nitroprusside (10-9-10-6 M) by cumulative addition of each agent to separate wells. Each agent was administered to a pair of rings, so that each ring was exposed to only one vasodilator agent.
cGMP experiments. Method. In this protocol, NO production was estimated by measuring accumulation of cGMP, the second messenger produced by vascular smooth muscle in response to NO. To this end, rings were incubated in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine using the method of Shaul et al.(18). Each ring was placed in an individual sealed polypropylene chamber containing 1 mL of oxygenated Krebs buffer. After 20 min, some rings were frozen in liquid N2 and stored at -70°C until cGMP analysis; cGMP in these samples reflected the endogenous vessel content before incubation. In the remaining segments incubation was initiated by replacing the existing buffer with 1 mL of Krebs buffer containing 3-isobutyl-1-methylxanthine (10-3 M). Incubation proceeded for either 0.5 or 1.0 min. At the end of incubation, the ring was removed and frozen in liquid N2, and 0.5 mL of cold trichloroacetic acid (21%) was added to the incubation medium; thereafter, the ring was returned to the medium. Combined analysis of vessel segment and incubation medium allowed the determination of newly produced cGMP within the tissue as well as that released into the incubation medium. Rings were homogenized within the incubation medium in ground glass pestles. Protein content was determined in duplicate aliquots of homogenate by the Lowry(23) method using BSA as a standard. The remaining homogenate was centrifuged at 1300 × g for 15 min. The supernatant underwent triplicate ether extraction and thereafter was dried at 40°C. cGMP was quantified by RIA after acetylation using commercial antibody (DuPont NEN, Boston, MA). RIA was run in triplicate on each sample.
Protocol. Basal, or unstimulated, cGMP accumulation was assessed by incubating rings in the absence of specific stimuli known to activate NO synthase or soluble guanylate cyclase. Thus, unstimulated cGMP accumulation was assumed consequent to activation of soluble guanylate cyclase by the continuous, basal production of NO known to occur in endothelial cells(10, 11). Stimulated cGMP production was determined by the addition of substance P (10-8 M) or A23187(10-7 M) to the chamber before the onset of incubation. The use of cGMP accumulation as a marker for NO production is contingent upon the assumption that endothelium-derived NO is acting as the sole stimulant for soluble guanylate cyclase within vascular smooth muscle. Three preliminary studies were carried out to confirm this assumption. First, to confirm that cGMP accumulation was contingent upon the presence of an intact endothelium, accumulation was compared between normal rings and rings subjected to mechanical destruction of the endothelial layer. This action was achieved by passing a heavy silk tie through the lumen several times, a method previously determined to remove the endothelium without damaging vascular smooth muscle(24). Second, to confirm that cGMP accumulation was contingent upon the presence of NO synthase, accumulation was compared between normal rings and rings pretreated with the arginine analogNG-monomethyl-L-arginine (10-4 M), an agent known to block NO production(25). Finally, to confirm that cGMP accumulation was contingent upon soluble guanylate cyclase, accumulation was compared between normal rings and rings pretreated with methylene blue (3× 10-5 M), an agent which blocks cyclase enzyme activity(26). These preliminary studies were carried out in a separate group of study subjects not used in the experimental protocols and observations were made during basal, or unstimulated conditions only.
Statistical analyses. All observations were made in a pair of mesenteric artery rings and the average response determined. In subsequent data presentation the term n refers to the number of subjects in which the observation was made. Data from the ring tension studies were analyzed by a 3-way ANOVA for repetitive measures which used group (3-versus 35-d-old), condition (control versus ischemia-reperfusion) and drug dose as main effects. Individual analyses were conducted for substance P, A23187, and nitroprusside data, inasmuch as these observations were made in discrete populations of rings. If the F statistic was significant (p < 0.01) post hoc tests were carried out to determine the sites of significant difference. Data for the cGMP accumulation experiments were treated in a similar fashion, with separate 3-way ANOVA carried out on basal, substance P, and A23187 data.
RESULTS
Hemodynamic data are presented in Tables 1 and 2. Baseline intestinal blood flow and vascular resistance were age-dependent; specifically, flow was greater and resistance lower in younger subjects. Induction of a mesenteric artery coarctation decreased intestinal blood flow to ≈10% of baseline, and this value remained low as long as the tie was left in place. Upon release of the tie, intestinal blood flow rapidly increased well above baseline in both age groups. In older subjects, the increased flow rate remained for the duration of the reperfusion period. In younger subjects, however, this hyperemic period was brief; within 15 min after release of the tie, intestinal blood flow had decreased below baseline where it remained for the duration of the alloted reperfusion period. Subjects in the control group maintained excellent hemodynamic stability throughout the duration of the in vivo portion of the experiment.
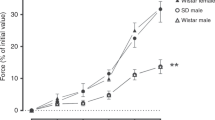
The ring tension experiments clearly indicate an age-dependent effect of ischemia-reperfusion on endothelial cell function. A similar trend was noted for substance P and A23187, the endothelium-dependent dilators(Figs. 1 and2). Mesenteric artery rings from 3-d-old subjects exposed to ischemia-reperfusion demonstrated significant attenuation in the degree of relaxation induced by substance P at concentrations ≥10-9 M and to A23187 at concentrations ≥5 × 10-7 M. In contrast, rings from older subjects subjected to ischemia-reperfusion demonstrated significant enhancement of relaxation to substance P at 10-7 M and to A23187 at concentrations greater ≥5× 10-8 M. Ischemia-reperfusion had no effect on relaxation in response to the endothelium-independent dilator sodium nitroprusside in either age group (Fig. 3).
The effects of substance P on isometric tension in postnatal mesenteric artery rings in vitro. Rings from 3-d-old subjects are shown as circles, whereas those from 35-d-old subjects are shown as triangles; closed symbols denote control rings, whereasopen circles indicate rings exposed to ischemia-reperfusionin vivo. The F statistic for the 3-way ANOVA was significant (p < 0.001). Subsequent 2-way ANOVA performed within each age group using condition (control vs ischemia-reperfusion) and substance P dose (10-11-10-7 M) as main effects were significant in both age groups (p < 0.001 in 3-d; p = 0.0433 in 35-d). Data are given as mean ± SEM, n = 8 for 35-d,n = 8 for 3-d; *p < 0.01 vs control.
The effects of A23187 on isometric tension in postnatal mesenteric artery rings in vitro. Rings from 3-d-old subjects are shown as circles, whereas those from 35-d-old subjects are shown astriangles; closed symbols denote control rings, whereas open circles indicate rings exposed to ischemia-reperfusion in vivo. The F statistic for the 3-way ANOVA was significant (p< 0.001). Subsequent 2-way ANOVA performed within each age group using condition (control vs ischemia-reperfusion) and A23187 dose(10-9-10-6 M) as main effects were significant in both age groups (p = 0.004 in 3-d; p < 0.001 in 35-d). Data are given as mean ± SEM, n = 8 for both age groups; *p< 0.01 vs control.
The effects of sodium nitroprusside on isometric tension in postnatal mesenteric artery rings in vitro. Rings from 3-d-old subjects are shown as circles, whereas those from 35-d-old subjects are shown as triangles; closed symbols denote control rings, whereas open circles indicate rings exposed to ischemia-reperfusion in vivo. The F statistic for the 3-way ANOVA was not significant. Data are given as mean ± SEM,n = 8 for 3-d-old, n = 8 for 35-d-old.
The preliminary studies confirmed that cGMP accumulation by in vitro mesenteric artery segments was dependent on the stimulation by endothelium-derived NO. Thus, basal cGMP accumulation was reduced 80 ± 5% after 1 min of incubation in rings subjected to mechanical removal of the endothelium. Similarly, pretreatment withNG-monomethyl-L-arginine (10-4 M) or methylene blue (3× 10-5 M) decreased basal cGMP accumulation at 1 min by 71± 5% and 62 ± 4%, respectively.
The effects of ischemia-reperfusion on cGMP accumulation in postnatal mesenteric artery were similar to those noted during the ring tension experiments. Mesenteric artery rings from both age groups demonstrated significant production of cGMP under basal, or unstimulated, conditions(Fig. 4). Ischemia-reperfusion decreased the basal accumulation rate in rings from younger subjects, but increased it in rings from 35-d-old subjects. Similarly, stimulated cGMP production was attenuated by ischemia-reperfusion in rings from 3-d-old subjects, but increased in older subjects (Figs. 5 and6). In control rings from younger subjects, both substance P and A23187 caused a brisk increase in cGMP production at 0.5 and 1.0 min of incubation; this effect was not observed in tissue subjected to ischemia-reperfusion. Mesenteric artery rings from 35-d-old subjects demonstrated a more modest degree of stimulated cGMP production; however, stimulated production in response to substance P and A23187 was greater in rings exposed to ischemia reperfusion than in control rings.
The cGMP accumulation in mesenteric artery rings from postnatal swine. Circles denote 3-d-old subjects, whereastriangles indicate 35-d-old subjects; closed symbols indicate control rings, whereas open symbols represent rings exposed to ischemia-reperfusion. These data represent basal, or unstimulated, accumulation. The F statistic for the 3-way ANOVA was significant(p = 0.008). Subsequent 2-way ANOVA performed within each group using condition (control vs ischemia-reperfusion) and time (0, 0.5, and 1 min) as main effects were significant in both groups (p < 0.001 in 3-day, p = 0.0367 in 35-d). Data are given as mean ± SEM, n = 8 for both groups, *p < 0.01 vs t = 0; †p = 0.01 vs control.
The cGMP accumulation in mesenteric artery rings from postnatal swine in response to stimulation by substance P. Circles denote 3-d-old subjects, whereas triangles indicate 35-d-old subjects; closed symbols indicate control rings, whereas open symbols represent rings exposed to ischemia-reperfusion. The F statistic for the 3-way ANOVA was significant (p < 0.001). Subsequent 2-way ANOVA performed within each group using condition (controlvs ischemia-reperfusion) and time (0, 0.5, and 1 min) as main effects were significant in both groups (p = 0.0063 in 3-d,p = 0.0101 in 35-d). Data are given as mean ± SEM,n = 8 for both groups, *p < 0.01 vs t = 0;†p = 0.01 vs control.
The cGMP accumulation in mesenteric artery rings from postnatal swine in response to stimulation by A23187. Circles denote 3-d-old subjects, whereas triangles indicate 35-d-old subjects;closed symbols indicate control rings, whereas open symbols represent rings exposed to ischemia-reperfusion. The F statistic for the 3-way ANOVA was significant (p = 0.0089). Subsequent 2-way ANOVA performed within each group using condition (controlvs ischemia-reperfusion) and time (0, 0.5, and 1 min) as main effects were significant in both groups (p = 0.0107 in 3-d,p = 0.0152 in 35-d). Data are given as mean ± SEM,n = 8 for both groups, *p < 0.01 vs t = 0;†p = 0.01 vs control.
DISCUSSION
The perturbation of ischemia-reperfusion had a qualitatively different effect on endothelial cell production of NO in mesenteric artery from 3- and 35-d-old subjects: production was attenuated in younger, but amplified in older subjects. The results of the in vitro assays corresponded nicely to the in vivo hemodynamic effects of ischemia-reperfusion observed during the pilot studies. Thus, intestinal vascular resistance noted at the completion of reperfusion was significantly greater than baseline in 3-d-old subjects, but well below baseline in older animals. Endothelial cell production of NO plays an important role in setting basal vascular tone in postnatal intestine(15, 24). Thus, compromise of NO production, as occurred in 3-d-old intestine after ischemia-reperfusion, might be expected in increase vascular resistance inasmuch as an important dilator stimulus is lost. The balance between constrictor and dilator stimuli which determines vascular resistance would be shifted in favor of constriction, assuming that other factors remained quantitatively unchanged.
Why did the two age groups respond to ischemia-reperfusion so differently? Unfortunately, the present data offer no clear insight into this question. It may be useful, however, to review the NO-cGMP pathway inasmuch as amplification and attenuation of this pathway appeared to occur in older and younger swine intestine, respectively, in response to ischemia reperfusion. The constitutive isoform of nitric oxide synthase (cNOS) within the endothelium is activated by a Ca2+-calmodulin complex, which in turn forms when cytosolic free Ca2+ increases. Activated cNOS oxidizes L-arginine to L-citrulline and in the process liberates NO; this reaction requires O2, and cofactors including NADPH, flavin nucleotides, as well as tetrahydrobiopterin. NO has a brief half-life (seconds) and can be inactivated by several factors, including superoxide anion and Hb during transit from its site of production (the endothelial cell) to its site of action (the vascular smooth muscle cell). NO exerts its principal vascular effect by binding to the iron in the heme group of soluble guanylate cyclase in adjacent vascular smooth muscle, an action which increases [cGMP](10, 11). How might the NO-cGMP pathway have been altered by ischemia-reperfusion? Several possibilities should be noted, based on work in other circulations. These include disruption of membrane-bound receptors which gate Ca2+ flux; loss of membrane integrity which would affect partitioning of Ca2+ stores; diminished cNOS-mRNA expression; loss of cofactor or substrate availability; increase in the presence of factors that bind released NO; diminished soluble guanylate cyclase; or damage to the contractile machinery within the vascular smooth muscle. Clearly, these possibilities are entirely speculative from the perspective of the present work.
Ischemia-reperfusion had no effect on the response of in vitro mesenteric artery rings to sodium nitroprusside in either age group. This observation makes it very unlikely that the age-dependent effects of ischemia-reperfusion were consequent to events within vascular smooth muscle. Sodium nitroprusside is a nitrovasodilator which directly stimulates soluble guanylate cyclase within vascular smooth muscle and its effect is fully independent of the endothelium(20). Soluble guanylate cyclase and the contractile machinery necessary for vascular smooth muscle contraction were clearly functional in both age groups after ischemia-reperfusion.
In contrast, ischemia-reperfusion significantly altered the response to substance P and A23187 in both groups, but in qualitatively different fashions. Substance P binds to a membrane receptor, and the resulting ligand-receptor complex increases Ca2+ flux into the endothelial cell; in contrast, A23187 directly increases Ca2+ flux without cell membrane binding(20). The response to both agents was attenuated in younger subjects. This observation suggests that ischemia-reperfusion-mediated damage was not limited to the endothelial cell membrane or membrane-bound receptors, but also involved the intracellular machinery necessary for NO production, including NOS, substrate, or cofactors. Alternatively, the fate of NO after its production might have been affected by ischemia-reperfusion. This notion is especially intriguing inasmuch as the concentration of superoxide anion might be substantially increased after ischemia-reperfusion, especially in the newborn, who generally has a limited ability to detoxify oxygen free radical species(26). Such a circumstance might significantly reduce the amount of endothelium-derived NO reaching its site of vascular action within adjacent vascular smooth muscle. Increased metabolism of NO would be evidenced by attenuation of ring tension relaxation and decreased cGMP production in the indirect measurement techniques used in these experiments. It is a bit more difficult to explain the increase in response to substance P and A23187 noted in older subjects. One interesting possibility is increased NOS-mRNA expression, an event which seems temporally feasible given the lag time between induction of ischemia in vivo and estimation of NO production in vitro. Such an occurrence makes physiologic sense: increased NO production, brought about by increased NOS activity, would serve to maximize intestinal vascular conductance during reperfusion and so enhance the conductive flux of oxygen from the macrocirculation to the intestinal capillaries. This action would assure rapid repayment of the oxygen debt incurred during ischemia. Clearly, however, all the aforementioned possibilities are mere speculation. At present it is possible to conclude only that the perturbation of ischemia-reperfusion had an age-dependent effect on the L-arginine-NO-cGMP pathway, as estimated by two indirect measures of NO production.
There exists precious little data regarding the circulatory physiology of the human newborn intestine, and acquisition of additional data with currently available technology is unlikely; therefore, animal models remain an essential means to evaluate specific circulatory mechanisms which might participate in the etiology of NEC. To suggest that events observed in newborn swine might play a role in the pathogenesis of NEC necessitates more assumptions than editors permit, even though there is likely more homology than dissimilarity between piglets and infants(19). This caveat affirmed, do the present data provide new insights into the relationship between the circulation and NEC?
The single contribution of these data is the recognition that the perturbation of ischemia-reperfusion has an age-dependent effect on vascular function within postnatal intestine. Specifically, this perturbation attenuated production of endothelium-derived NO, an agent which is a major vasodilator stimulus and also participates in preventing platelet and neutrophil adhesion to the endothelial surface(10, 11). Loss of NO production is an important determinant in the development of local vasoconstriction, thrombosis, and sustained ischemia in the coronary circulation(27, 28); certainly, a similar phenomenon might occur within the newborn intestinal circulation and participate in the development of gut necrosis. Thus, events such as the ischemia-reperfusion cycle might exert their principal effect on the vascular endothelium, not the parenchyma, leading to a regional vasculopathy which would compromise perfusion is a more subtle, long-standing fashion. Stated otherwise, the relevant ischemia during the development of NEC would be secondary to dysfunction of the intestinal vasculature and would occur within small regions of the intestine, possibly even within discrete layers of the gut wall. This approach differs from that traditionally held, which envisions a global ischemia imposed on the entire intestine.
This hypothesis is especially attractive inasmuch as factors other than ischemia-reperfusion might initiate endothelial cell damage. For example, bacterial endotoxin(29) and platelet-activating factor(30), two agents implicated in the pathogenesis of NEC, might exert part of their deleterious effects via induction of endothelial cell damage. Similarly, cytokines released from mucosal cells injured by luminal irritants(31) might damage the endothelium; the resulting ischemia would then be an important secondary process in the development of NEC. In this sense, endothelial cell dysfunction might represent a common mechanism by which a number of different “trigger events” could eventually lead to significant intestinal necrosis. Finally, the hypothesis fits the unique epidemiology of NEC inasmuch as significant endothelial cell dysfunction was noted only in younger subjects.
Abbreviations
- NEC:
-
necrotizing enterocolitis
- NOS:
-
nitric oxide synthase
- NO:
-
nitric oxide
- ANOVA:
-
analysis of variance
References
Lloyd JR 1969 The etiology of gastrointestinal perforations in the newborn. J Pediatr Surg 4: 77–84
Touloukian RJ, Posch JN, Spencer R 1972 The pathogenesis of gastroenterocolitis of the neonate: selective gut mucosal ischemia in asphyxiated neonatal piglets. J Pediatr Surg 7: 194–204
Alward CT, Hook JB, Helmrath TA, Matson JC, Baile MD 1977 Effects of asphyxia on cardiac output and organ blood flow in the newborn piglet. Pediatr Res 12: 824–827
Kliegman RM, Hack M, Jones P, Fanaroff AA 1982 Epidemiologic study of necrotizing enterocolitis among low birth weight infants; absence of identifiable risk factors. J Pediatr 100: 440–444
Stoll BJ, Kanto WP, Glass RI, Nahmias AJ, Brann AW 1980 Epidemiology of necrotizing enterocolitis: a case control study. J Pediatr 96: 447–451
Hopkins GB, Gould VE, Stevenson JK, Oliver TK 1970 Necrotizing enterocolitis in preterm infants: a clinical and pathologic evaluation of autopsy material. Am J Dis Child 120: 229–232
Tait RA, Kealy WF 1979 Neonatal necrotizing enterocolitis. J Clin Pathol 32: 1090–1099
Bunton GL, Durbin GM, McIntosh N, Shaw DG, Taghizadeh A, Reynolds EOR, Rivers PA, Urman G 1977 Necrotizing enterocolitis: controlled study of 3 years' experience in a neonatal intensive care unit. Arch Dis Child 52: 772–777
Leake RD, Thanopoulos B, Nieberg R 1975 Hyperviscosity syndrome associated with necrotizing enterocolitis. Am J Dis Child 129: 1192–1194
Moncada S, Higgs A 1993 The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012
Vanhoutte P 1989 Endothelium and control of vascular function: state of the art lecture. Hypertension 13: 658–667
Ambrosio G, Weismann HF, Mannisi JA, Becker LC 1989 Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 80: 1846–1861
VanBenthuysen KM, McMurtry IF, Horwitz LD 1987 Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractilityin vitro. J Clin Invest 79: 265–279
Tsao PS, Lefer AM 1990 Time course and mechanism of endothelial dysfunction in isolated ischemic- and hypoxic-perfused rat hearts. Am J Physiol 259:H1660–H1666
Nowicki PT, Edwards RE 1992 Effect ofNG-monomethyl-L-arginine on postnatal intestinal vascular resistance is age-dependent. Pediatr Res 31: 64A( abstr)
Nowicki PT, Nankervis CA, Miller CE 1993 Effects of ischemia and reperfusion on intrinsic vascular regulation in the postnatal intestinal circulation. Pediatr Res 33: 400–404
Seccombe JF, Schaff HV 1994 Experimental methods in the study of endothelial function. In: Seccombe JF, Schaff HV (eds) Vasoactive Factors Produced by the Endothelium. RG Landis Co., Austin, TX, pp 27–42
Shaul PW, Farrar MA, Zellers TM 1992 Oxygen modulates endothelium-derived relaxing factor production in fetal pulmonary arteries. Am J Physiol 262:H355–H364
Douglas WR 1971 Of pigs and men and research: a review of the applications of the pig. Sus scrofa in human medical research. Space Life Sci 3: 226–234
Furchgott RF 1983 Role of endothelium in responses of vascular smooth muscle. Circ Res 53: 557–573
Deleted in proof
Deleted in proof
Lowry OH, Rosebrough NJ, Farr AL 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Nankervis CA, Nowicki PT 1995 The role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol ( in press)
Rees DD, Palmer RMJ, Hodson HF, Moncada S 1989 A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol 96: 418–424
Granger DN, Rutili G, McCord JM 1982 Superoxide radicals in feline intestinal ischemia. Gastroenterology 181: 22–29
Vanhoutte PM, Shimokawa H 1989 Endothelium-derived relaxing factor and coronary artery vasospasm. Circulation 80: 1–9
Luscher TF 1989 Endothelium-derived relaxing factor and contracting factors: potential role in coronary artery disease. Eur Heart J 10: 847–857
Fasano A, Kay BA, Russell RG 1990 Enterotoxin and cytotoxin production by enteroinvasive Escherichia coli. Infect Immunol 58: 3717–3721
Hseuh W, Gonzalez-Crussi R, Arroyave J 1986 Platelet activating factor induced ischemic bowel necrosis. Am J Pathol 122: 231–236
Clark DA, Thompson JE, Schneider AJ, Weiner J, McMillan A, Rokahr J 1985 Necrotizing enterocolitis-intraluminal biochemistry in human infants and a rabbit model. Pediatr Res 19: 919–921
Acknowledgements
I thank David Dunaway and Charles Miller for their excellent technical assistance and Mary Smith for secretarial support. I also thank Drs. Thomas Zellers and Philip Shaul of the University of Texas, Southwestern Medical School, and Dr. Joseph Benoit of the Louisiana State University, Shreveport for teaching me the methods used in this work.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grant HD 31902-01.
Rights and permissions
About this article
Cite this article
Nowicki, P. The Effects of Ischemia-Reperfusion on Endothelial Cell Function in Postnatal Intestine. Pediatr Res 39, 267–274 (1996). https://doi.org/10.1203/00006450-199602000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199602000-00014