Abstract
Background
Acetaminophen is widely prescribed to both neonates and young children for a variety of reasons. In adults, therapeutic usage of acetaminophen induces systemic arterial pressure changes and exposure to high doses promotes tissue toxicity. The pulmonary vascular effects of acetaminophen at any age are unknown. Hypothesizing that, early in life, it promotes vasomotor tone changes via oxidative stress, we tested the in vitro acetaminophen effects on intrapulmonary and carotid arteries from newborn and adult rats.
Method
We measured the acetaminophen dose–response in isometrically mounted arteries and pharmacologically evaluated the factors accounting for its vasomotor effects.
Results
Acetaminophen induced concentration- and age-dependent vasomotor tone changes. Whereas a progressive increase in vasomotor tone was observed in the newborn, the adult arteries showed mostly vasorelaxation. Inhibition of endogenous nitric oxide generation with l-NAME and the use of the peroxynitrite decomposition catalyst FeTPPS (Fe(III)5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato chloride) mostly abolished the drug-induced increase in newborn pulmonary vasomotor tone
Conclusions
In newborn rats, acetaminophen increases pulmonary vasomotor tone via peroxynitrite generation. Given its therapeutic usage, further clinical studies are warranted to assess the acetaminophen effects on the newborn pulmonary and systemic vascular resistance.
Similar content being viewed by others
Introduction
Acetaminophen has analgesic, antipyretic, and anti-inflammatory properties, which makes it the most frequently used medication, across all ages.1 Recently, the effect of acetaminophen on promoting closure of the ductus arteriosus was recognized extending its therapeutic use to neonates with persistent vessel patency, leading to increased transductal flow and its hemodynamic disturbances.2
Although considered a relatively safe drug, severe hepatic and renal toxicity is observed following acute exposure to an excessive acetaminophen dose.3 Even when administered within the recommended therapeutic dosage, its use is associated with changes in systemic arterial pressure in adult subjects.4,5,6,7 There are limited data on the acetaminophen-induced systemic blood pressure changes in children, but the effects appear to be similar, when compared with adults.8,9,10,11
Interestingly, only the acetaminophen-related vascular effect on the systemic circulation has been evaluated at any age. Whether this drug can equally alter pulmonary vascular tone is currently unknown, but there is no reason to expect such effect to be unique to the systemic vasculature.
The pulmonary vascular resistance is relatively high in neonates, when compared with the postnatal period and further exacerbated in the presence of lung disease.12 It is plausible that, early in life, acetaminophen may have either harmful or beneficial clinical effects. A further increase in right ventricular afterload would be harmful by further reducing lung blood flow and facilitating right-to-left shunting via the foramen ovale, or the ductus arteriosus. Yet, acetaminophen-induced pulmonary vasoconstriction may decrease the magnitude of the transductal shunt, thus facilitating its closure.
Therefore, the purpose of the present study was to evaluate, in vitro, the acetaminophen-induced age- and circulation-dependent vasomotor changes. We hypothesized that, early in life, acetaminophen promotes vasomotor tone changes in systemic as well as pulmonary vessels.
Materials and methods
Animals
Sprague–Dawley and Wistar rats were studied. All procedures were conducted according to the criteria established by the Canadian Council on Animal Care and were approved by the Animal Care Committee of The Hospital for Sick Children Research Institute.
Organ bath studies and Fulton index determination
Rats were studied at 2–7 (newborn, N = 110) and 60–90 (adult, N = 14) days of age. The animals were sacrificed with an overdose of pentobarbital sodium (50 mg/kg, intraperitoneally (i.p.)). The lungs and carotid arteries were quickly removed and maintained on an ice bed for further dissection. The animals’ sex was recorded to enable characterization of any sex-dependent effect.
The near-resistance (3rd–4th generations) intrapulmonary arteries were dissected free and mounted on a wire myograph (Danish Myo Technology A/S, Aarhus, Denmark). A similar setup was used for the carotid arteries. The muscle bath was filled with Krebs–Henseleit buffer solution (NaCl, 115 mM; NaHCO3, 25 mM; NaHPO4, 1.38 mM; KCl, 2.51 mM; MgSO4·7H2O, 2.46 mM; CaCl2, 1.91 mM; and dextrose, 5.56 mM), bubbled with air/6% CO2 and maintained at 37 °C. After 1 h of equilibration, the optimal vessel resting tension was determined by repeated stimulation with 128 mM KCl until maximum active tension was reached. All subsequent force measurements were obtained at optimal resting tension.
Acetaminophen dose–response (10−9–10−4 M) effect on the arteries’ vasomotor tone was obtained. Preliminary experiments showed that neither pulmonary nor carotid arteries developed significant tone in response to acetaminophen alone. For this reason, the vessels were pre-stimulated with the thromboxane A2 mimetic U46619 (newborn: 10−6 M; adult 10−8 M), prior to acetaminophen exposure. The rationale for this was based on our previously recognized need to raise their basal tone when studying the peroxynitrite-induced pulmonary vascular effects.13 The acetaminophen dose–response was expressed as a percentage of the U46619-induced force. Contractile responses were normalized to the tissue cross-sectional area as follows: (width × diameter) × 2 and expressed as mN/mm2.
Unless otherwise noted, all utilized chemicals and drugs were purchased from Sigma (Oakville, ON, Canada).
Statistical methods
Data were first evaluated to determine Gaussian distribution by Skewness, Kurtosis, and Omnibus testing to confirm normal distribution. The data were analyzed by repeated-measures two-way analysis of variance with multiple comparisons obtained by the Tukey–Kramer test. Statistical significance was determined at P < 0.05. Only two (right and left) pulmonary, or carotid vessels per animal were studied. The sample size refers to the number of vessels studied. At least four vessels were used at each postnatal age (newborn versus adult) and vascular bed (pulmonary versus systemic) were used for the different experiments. All statistical analyses were performed with the Number Cruncher Statistical System software (NCSS, Kaysville, UT, USA). Data are presented as means ± SEM.
Results
Acetaminophen induces vasomotor tone in newborn pulmonary and systemic arteries
In response to acetaminophen, Sprague–Dawley newborn rats’ pulmonary arteries showed increased motor tone in a dose-dependent manner (Fig. 1). At the highest tested concentration (10−4 M), the acetaminophen-induced response was three-fold greater in female tissue, when compared with male-derived vessels (Fig. 1). Experiments conducted in same age Wistar rats’ pulmonary arteries showed similar results, when compared with Sprague–Dawley data, indicating that the acetaminophen-induced vasomotor tone was not strain-specific (Fig. 1). To evaluate whether the acetaminophen-induced pulmonary vasocontraction was dependent on U46619 pre-stimulation, we obtained a dose–response curve following prostaglandin E2α (PGF2α) (10−5 M) exposure. Acetaminophen induced a similar magnitude of sex-dependent increase in pulmonary vasomotor tone (data not shown).
Data (mean ± SE) expressed as a percentage of the force obtained following pre-stimulation with the thromboxane A2 analog U46619 (10−6 M). Vessel sample size: SD (female: N = 17 and male: N = 16) and Wistar (female: N = 8 and male: N = 16). **P < 0.001 as compared with arteries from female animals of either strain by repeated-measures two-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparison testing.
We next evaluated the acetaminophen dose–response in newborn carotid arteries. As shown in Fig. 2a, comparable acetaminophen-induced dose–response pattern was seen in the carotid arteries and the magnitude of vasomotor changes was equally sex-dependent. All subsequent experiments were conducted in arteries from female animals of both strains to eliminate sex as a confounding factor.
Data (mean ± SE) are expressed as a percentage of the force obtained following pre-stimulation with the thromboxane A2 analog U46619 (10−6 M). Vessel sample size: pulmonary: (female: N = 31 and male: N = 23) and carotid arteries (female: N = 11 and male: N = 7). **P < 0.001 as compared with female animals’ pulmonary, or carotid arteries by repeated-measures two-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparison testing.
Age-dependent differences in acetaminophen-induced vasomotor tone
An age comparison of the acetaminophen dose–response in pulmonary and carotid arteries showed that the magnitude of force increase was highest early in life, for both vessels (Fig. 3). When compared with the dose-dependent higher vasomotor tone observed in newborn pulmonary and carotid arteries, adult vessels exhibited vasocontraction at lower doses, followed by vasorelaxation at the highest tested concentration (Fig. 3).
Acetaminophen dose–response in newborn (2–7 days) and adult (>60 days of age) female rats (both strains) near-resistance pulmonary (a) and carotid arteries (b). Data are (mean ± SE) expressed as a percentage of the force obtained following pre-stimulation with the thromboxane A2 analog U46619 (10−6 M for newborn and 10−8 M for adult arteries). Vessel sample size: pulmonary (newborn: N = 31 and adult: N = 17) and carotid arteries (newborn: N = 12 and adult: N = 6). **P < 0.001 as compared with newborn pulmonary and carotid arteries by repeated-measures two-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparison testing.
Signal transduction pathway responsible for the acetaminophen-induced vasomotor tone
We evaluated the signal transduction pathway, which accounts for acetaminophen-induced pulmonary vasocontraction in newborn arteries. First, as tissue toxicity is dependent on reduced glutathione availability,3 we tested the effect of a reactive oxygen species scavenger on the pulmonary vasomotor tone changes induced by acetaminophen. The presence of excess glutathione in the muscle bath (10−2 M) prevented acetaminophen-induced increased tone and promoted pulmonary vasorelaxation in newborn tissue (Fig. 4a).
a Reduced glutathione (GSH; 10−2 M; N = 4); b superoxide scavengers Tempol (10−3 M; N = 5) and Mito-Tempo (10−5 M; N = 5), c peroxynitrite decomposition catalyst FeTPPS (10−4 M; N = 4); and d nitric oxide synthase inhibitor l-NAME (10−4 M; N = 9). Data (mean ± SE) expressed as a percentage of the force obtained following pre-stimulation with the thromboxane A2 analog U46619 (10−6 M). **P < 0.001 as compared with control data by repeated-measures two-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparison testing.
We then utilized the superoxide scavenger, Tempol (10−3 M), and its intracellular mitochondrial counterpart, Mito-Tempo (10−5 M), to evaluate their effects on acetaminophen-induced changes in pulmonary vasomotor tone. Both superoxide scavengers significantly reduced acetaminophen-induced pulmonary arterial vasocontraction from newborn animals (Fig. 4b).
We proceeded to investigate whether the acetaminophen-induced pulmonary vasomotor tone effect was peroxynitrite-mediated in newborn rats. The rationale for these experiments relates to our published data demonstrating that, similar to the present acetaminophen response pattern, peroxynitrite induced age-dependent changes in pulmonary vasomotor tone.13 As shown in Fig. 4c, FeTPPS (Fe(III)5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato chloride; 10−4 M), a peroxynitrite decomposition catalyst, suppressed most acetaminophen-induced vasocontraction.
Since peroxynitrite generation depends on nitric oxide availability, we evaluated the effect of l-NAME, a non-specific nitric oxide synthase inhibitor, on acetaminophen responsiveness. In the presence of l-NAME (10−4 M), the acetaminophen-induced increase in pulmonary vasomotor tone of newborn tissue was suppressed (Fig. 4d). Together, these data suggest that acetaminophen increases pulmonary vasomotor tone via peroxynitrite generation.
Finally, we evaluated the effect of sex on the magnitude of acetaminophen-induced vasomotor changes. We previously reported that the pattern of U46619 dose–response changes, following hyperoxia exposure in newborn rats, was sex-dependent.14 Considering that acetaminophen-induced increased tone in the newborn pulmonary arteries required pre-stimulation with U46619, we investigated whether the sex-dependent differences were related to this agonist instead. We, therefore, evaluated the sex-dependent differences in the newborn pulmonary vasomotor response to U46619, under the same conditions as used for acetaminophen studies. As shown in Fig. 5a, pulmonary arteries from female pups showed a left shift in the force dose–response curve that was suppressed in the presence of FeTPPS (10−4 M; Fig. 5b).
Thromboxane A2 analog U46619 dose–response (normalized to the response to KCl 128 mM induced force) in pulmonary arteries from female and male pups in the absence (a; N = 9 and 12, respectively) and presence of the peroxynitrite decomposition catalyst FeTPPS (10−4 M) (b; N = 5 and 4, respectively). **P < 0.001 as compared with female data by repeated-measures two-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparison testing.
Discussion
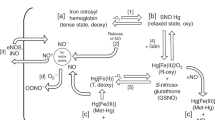
In the current study, we documented, in vitro, that acetaminophen induces age- and concentration-dependent pulmonary and systemic vasomotor changes. Whereas acetaminophen induced an increase in the newborn rat pulmonary and carotid arteries, vasomotor tone and vasorelaxation was mostly observed in comparable vessels in adult animals. Experiments to characterize the mechanism of acetaminophen-induced increase in pulmonary vasomotor tone in newborn rats showed that peroxynitrite generation was the likely response mediator. Figure 6 illustrates the proposed signal transduction pathway accounting for the acetaminophen-induced vascular changes in newborn arteries.
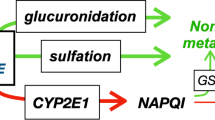
Acetaminophen, when administered in therapeutic doses to mammalians, is cleared via glucuronidation, sulfation, and renal excretion.15 In very preterm infants, acetaminophen glucuronidation is low and increases with gestational age.9 When compared with adult data, a significant increase in exposure to sulfate, cysteine and mercaptopurine metabolites was documented in preterm infants following intravenous acetaminophen administration.10
In humans, <5% of acetaminophen participates, via the cytochrome P450 2E1 isoenzyme metabolism, in the formation of a highly toxic compound called N-acetyl-p-benzoquinoneimine (NAPQI).16 NAPQI is an electrophilic reactive metabolite. When enough glutathione is available, NAPQI combines with this molecule and is inactivated. Tissue concentrations of NPAPQI exceeding the glutathione scavenging potential result in the formation of covalent adducts with nucleophilic thiol protein groups, which promotes cellular damage.17 Such toxicity is commonly observed following acetaminophen overdosing in adults and involves the liver, kidneys, and auditory cells.18,19,20 Acetaminophen-related tissue toxicity can also occur in neonates, and in a recently reported case, it was associated with liver tissue injury.21
Acetaminophen-induced vascular tissue injury has not been previously reported. Yet, the multiple reports linking acetaminophen therapeutic use with systemic blood pressure changes suggest that this drug may have a direct effect on vascular tissue.4,7,22 One systematic review of the effect of acetaminophen on blood pressure in adult subjects reported mixed results.5 Acetaminophen was associated with a decrease in systemic blood pressure 30 min when administered as an intravenous infusion,7 whereas a significant increase in pressure was documented in adult subjects following chronic use.22 In a cohort of healthy adult volunteers, a single dose of intravenous acetaminophen resulted in a transient decrease in the subjects’ systemic blood pressure, which further supports its vasodilator effects on the systemic vascular bed.23
There are limited data available on the potential hemodynamic effects of acetaminophen in children and neonates. A decrease of 3 mmHg in mean systemic blood pressure was reported in neonates receiving acetaminophen, 10–20 mg/kg intravenously, for analgesia.11
A large study in children with congenital heart disease showed that acetaminophen was associated with more frequent hypotension episodes.8 Clinically relevant systemic hypotension was documented in 5% and a decrease in mean arterial pressure, from basal level, in 20% of the reported subjects.8 Interestingly, skin temperature measurements of treated patients in the referred study8 suggest that the acetaminophen-induced hypotension was secondary to systemic vasodilatation. Finally, a recent metaanalysis concluded that intravenous acetaminophen is associated with clinically relevant hypotension in both critically ill children and adult subjects, and these risks should be considered when treating this population.24
Acetaminophen has recently been recognized as capable of reducing the ductus arteriosus blood flow and thus of therapeutic benefit in the treatment of its patency early in life.2 It is important to characterize the biological effects as the practice of acetaminophen use to close a patent ductus arteriosus in premature infants has grown substantially over the past 5–10 years.25,26
The mechanism accounting for this acetaminophen effect on the ductus arteriosus is unclear, but speculated to be related to its vasoconstrictive effect.27 In the present study, we documented that acetaminophen has a direct, age-dependent, effect on the rat vasomotor tone. A concentration-dependent increase in pulmonary and systemic vasomotor tone was observed in newborn-derived tissue, whereas vasorelaxation at higher drug concentrations was documented in the adult vessels. Interestingly, the in vitro carotid vasorelaxation shown in adult rats is consistent with acetaminophen-induced transient hypotension documented in healthy adult volunteers.23
Vascular peroxynitrite formation following acetaminophen administration to rodents was previously demonstrated.28 The present data suggest that NAPQI-dependent peroxynitrite generation is responsible for acetaminophen-induced changes in pulmonary vasomotor tone. The evidence in support of this speculation is as follows. Peroxynitrite formation depends on superoxide and nitric oxide availability. In the present study, we showed that superoxide dismutase inhibition with Tempol extracellularly, and intramitochondrial with Mito-Tempo significantly suppressed the acetaminophen-induced pulmonary vasomotor tone increase. In addition, we showed that l-NAME, a non-specific nitric oxide synthase inhibitor, completely suppressed acetaminophen-induced changes in newborn pulmonary vasomotor tone. Other investigators have shown that superoxide scavengers16 and nitric oxide synthase inhibition29 reduces acetaminophen hepatoxicity, suggesting that peroxynitrite actively participates in this process. Most importantly, in the presence of the peroxynitrite decomposition catalyst FeTPPS, we observed complete suppression of this response. Together, these data indicate that the acetaminophen-induced pulmonary vasomotor tone changes are mediated via peroxynitrite.
The acetaminophen-induced pulmonary vasomotor age- and sex-dependent response pattern merits discussion. In response to acetaminophen exposure, an increase in force was documented in newborn arteries, while adult vessels showed a relatively minor increase in force at lower concentration and vasorelaxation at the higher levels. Such a response pattern is comparable with our previously reported findings in rat pulmonary arteries exposed to peroxynitrite; specifically, we demonstrated increased vasomotor tone in newborn vessels, but decreased tone in adult counterparts.13 In addition, we documented an increase in 8-isoprostane production, following exposure to peroxynitrite in newborn, but not in adult pulmonary arterial tissue.13 The latter is important as we have previously shown that 8-isoprostane is a pulmonary vasoconstrictor in newborn rats.30 In rat pulmonary arteries, we demonstrated that 8-isoprostane induces endothelin expression, via the RhoA/ROCK pathway. Both agents promote an increase in pulmonary vasomotor tone, via distinct pathways. Endothelin has a direct pulmonary vasoconstrictor effect, whereas stimulation of the RhoA/ROCK pathway interferes with vasomotor relaxation.31 Finally, other investigators have reported acetaminophen dose in vivo results in reduced endothelium-dependent response and endothelium-independent relaxation response in adult rat aorta tissue in vitro.32
The observed sex-dependent discordance in the magnitude of acetaminophen-induced vasocontraction in newborn vessels is intriguing. Lee et al.33 reported that the prevalence of an adverse hypotensive effect, in adult subjects receiving acetaminophen, was sex-dependent and increased in men. To the best of our knowledge, there are no other published data suggesting a sex difference in the acetaminophen-induced hemodynamic changes.
To further characterize this effect, we investigated whether the observed sex differences were directly related to acetaminophen, or the thromboxane A2 analog U46619 utilized to pre-contract the pulmonary arteries before exposure to acetaminophen. Previous studies have shown that U46619 and PGF2α promote pulmonary arterial tissue superoxide generation via upregulation of NADPH (nicotinamide adenine dinucleotide phosphate) oxidase expression/activity.34 In this study, we demonstrated greater vasomotor tone in arteries derived from female pups, after U46619 stimulation, when compared with males. Thus, it is likely that the sex difference in the magnitude of the acetaminophen-induced vasocontraction was related to the U46619-induced superoxide generation and sex differences in oxidative stress-dependent vasomotor changes.
The present study has inherent limitations. First, acetaminophen-induced vasomotor changes demonstrated in vitro are not reflective of the in vivo environment where diverse confounding or competing factors may be at play. Yet, care was taken to restrict the tested muscle-bath acetaminophen concentrations to clinically relevant therapeutic levels. The target plasma concentration of acetaminophen for children and adults ranges between 10 and 100 µmol/L (10−5–10−4 M).35,36 These acetaminophen plasma levels correspond to the highest in vitro concentrations evaluated in the present study, thus lending further support to the clinical significance of our findings.
Whether the pulmonary vascular resistance increases following acetaminophen administration to neonates is unknown and requires further evaluation. To the best of our knowledge, only one study reported data potentially related to this issue. Oncel et al.37 described a single case of pulmonary hypertension in a preterm infant receiving ibuprofen, while none was noted following oral acetaminophen administration for ductal closure.
In conclusion, acetaminophen exposure, in vitro, promotes pulmonary and systemic vasomotor changes in rodents, likely via NAPQI formation and the resulting peroxynitrite generation. Given the current experimental findings, obtaining echocardiographic assessment of pulmonary vascular changes and systemic blood pressure monitoring in infants receiving acetaminophen are warranted. The present work was supported by funds from an operating grant from the Canadian Institutes of Health Research (J.B.; MOP 133664).
References
Cuzzolin, L., Antonucci, R. & Fanos, V. Paracetamol (acetaminophen) efficacy and safety in the newborn. Curr. Drug Metab. 14, 178–185 (2013).
Hammerman, C. et al. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics 128, e1618–e1621 (2011).
Mirochnitchenko, O. et al. Acetaminophen toxicity. Opposite effects of two forms glutathione peroxidase. J. Biol. Chem. 274, 10349–10355 (1999).
Dawson, J. et al. Acetaminophen use and change in blood pressure in a hypertensive population. J. Hypertens. 31, 1485–1490 (2013). discussion 1490.
Turtle, E. J., Dear, J. W. & Webb, D. J. A systematic review of the effect of paracetamol on blood pressure in hypertensive and non-hypertensive subjects. Br. J. Clin. Pharm. 75, 1396–1405 (2013).
Yadav, J. Can paracetamol raise blood pressure. J. Indian Med. Assoc. 111, 135 (2013).
Cantais, A. et al. Acetaminophen-induced changes in systemic blood pressure in critically Ill patients: results of a Multicenter Cohort Study. Crit. Care Med. 44, 2192–2198 (2016).
Achuff, B.-J. et al. Hypotensive response to IV acetaminophen in pediatric cardiac patients. Pediatr. Crit. Care Med. 20, 527–533 (2019).
Krekels, E. H. et al. Developmental changes rather than repeated administration drive paracetamol glucuronidation in neonates and infants. Eur. J. Clin. Pharmacol. 71, 1075–1082 (2015).
Flint, R. B. et al. Exposure to acetaminophen and all its metabolites upon 10, 15, and 20 mg/kg intravenous acetaminophen in very-preterm infants. Pediatr. Res. 82, 678 (2017).
Allegaert, K. & Naulaers, G. Haemodynamics of intravenous paracetamol in neonates. Eur. J. Clin. Pharm. 66, 855–858 (2010).
Fuloria, M. & Aschner, J. L. Persistent pulmonary hypertension of the newborn. Semin. Fetal Neonatal Med. 22, 220–226 (2017).
Belik, J., Jankov, R. P., Pan, J. & Tanswell, A. K. Peroxynitrite inhibits relaxation and induces pulmonary artery muscle contraction in the newborn rat. Free Radic. Biol. Med. 37, 1384–1392 (2004).
Enomoto, M. et al. Sex-dependent changes in the pulmonary vasoconstriction potential of newborn rats following short-term oxygen exposure. Pediatr. Res. 72, 468–478 (2012).
Anderson, B. J. Paracetamol (Acetaminophen): mechanisms of action. Paediatr. Anaesth. 18, 915–921 (2008).
Jaeschke, H. & Ramachandran, A. Oxidant stress and lipid peroxidation in acetaminophen hepatotoxicity. React. Oxyg. Species (Apex) 5, 145–158 (2018).
Jozwiak-Bebenista, M. & Nowak, J. Z. Paracetamol: mechanism of action, applications and safety concern. Acta Pol. Pharm. 71, 11–23 (2014).
Ahmed, O. G. & El-Mottaleb, N. A. Renal function and arterial blood pressure alterations after exposure to acetaminophen with a potential role of Nigella sativa oil in adult male rats. J. Physiol. Biochem. 69, 1–13 (2013).
Ito, Y., Abril, E. R., Bethea, N. W. & McCuskey, R. S. Role of nitric oxide in hepatic microvascular injury elicited by acetaminophen in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G60–G67 (2004).
Kalinec, G. M. et al. Acetaminophen and NAPQI are toxic to auditory cells via oxidative and endoplasmic reticulum stress-dependent pathways. Hear Res. 313, 26–37 (2014).
Walls, L., Baker, C. F. & Sarkar, S. Acetaminophen-induced hepatic failure with encephalopathy in a newborn. J. Perinatol. 27, 133–135 (2007).
Sudano, I. et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation 122, 1789–1796 (2010).
Chiam, E. et al. The haemodynamic effects of intravenous paracetamol (acetaminophen) in healthy volunteers: a double-blind, randomized, triple crossover trial. Br. J. Clin. Pharm. 81, 605–612 (2016).
Maxwell, E. N., Johnson, B., Cammilleri, J. & Ferreira, J. A. Intravenous acetaminophen–induced hypotension: a review of the current literature. Ann. Pharmacother. 53, 1033–1041 (2019).
Terrin, G. et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 101, F127–F136 (2016).
Jasani, B., Weisz, D. E. & McNamara, P. J. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Semin. Perinatol. 42, 243–252 (2018).
El-Khuffash, A. et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatr. Res. 76, 238 (2014).
Knight, T. R. et al. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol. Sci. 62, 212–220 (2001).
Hinson, J. A. et al. Effect of inhibitors of nitric oxide synthase on acetaminophen-induced hepatotoxicity in mice. Nitric Oxide 6, 160–167 (2002).
Belik, J. et al. Chronic O2 exposure in the newborn rat results in decreased pulmonary arterial nitric oxide release and altered smooth muscle response to isoprostane. J. Appl. Physiol. (1985) 96, 725–730 (2004).
Montani, D. et al. Targeted therapies in pulmonary arterial hypertension. Pharm. Ther. 141, 172–191 (2014).
Porto, H. K. P. et al. Exposure to acetaminophen impairs vasodilation, increases oxidative stress and changes arterial morphology of rats. Arch. Toxicol. 93, 1955–1964 (2019).
Lee, H. Y. et al. Propacetamol poses a potential harm of adverse hypotension in male and older patients. Pharmacoepidemiol. Drug Saf. 26, 256–264 (2017).
Muzaffar, S. et al. Iloprost inhibits NADPH oxidase expression and superoxide release in porcine pulmonary arteries and cells stimulated with thromboxane A2, isoprostane F2α and cytokines. Br. J. Pharm. 141, 488–496 (2004).
Gibb, I. A. & Anderson, B. Paracetamol (acetaminophen) pharmacodynamics: interpreting the plasma concentration. Arch. Dis. Child. 93, 241–247 (2008).
Jibril, F., Sharaby, S., Mohamed, A. & Wilby, K. J. Intravenous versus oral acetaminophen for pain: systematic review of current evidence to support clinical decision-making. Can. J. Hosp. Pharm. 68, 238 (2015).
Oncel, M. Y. et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J. Pediatr. 164, e511 (2014).
Author contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: L.T.H., P.J.M., and J.B. Drafting the article or revising it critically for important intellectual content: L.T.H., P.J.M., and J.B. Final approval of the version to be published: I.T.H., J.P., P.J.M., and J.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamir Hostovsky, L., Pan, J., McNamara, P.J. et al. Acetaminophen increases pulmonary and systemic vasomotor tone in the newborn rat. Pediatr Res 87, 1171–1176 (2020). https://doi.org/10.1038/s41390-019-0725-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0725-9
This article is cited by
-
Clinical and echocardiography predictors of response to first-line acetaminophen treatment in preterm infants with hemodynamically significant patent ductus arteriosus
Journal of Perinatology (2024)
-
Environmental toxicant-mediated cardiovascular diseases: an insight into the mechanism and possible preventive strategy
Toxicology and Environmental Health Sciences (2023)
-
Acetaminophen treatment evokes anticontractile effects in rat aorta by blocking L-type calcium channels
Pharmacological Reports (2022)
-
The unintended consequences of acetaminophen use for ductal closure in premature infants
Pediatric Research (2020)