Abstract
Background:
Few studies have investigated the impact of acute rhinosinusitis on disease-specific quality of life, and disease costs have not been studied previously in Scandinavia.
Aims:
To study symptoms, treatment patterns, quality of life and costs in adults with acute rhinosinusitis.
Methods:
This was an observational study in primary care. Patients aged 18–80 years seeking care for acute rhinosinusitis were evaluated using the Major Symptom Score (MSS) on days 0 and 15. Recommended and used treatments, quality of life and costs were assessed by questionnaires including EQ-5D™ and a visual analogue scale (VAS) on the same days.
Results:
150 patients were enrolled; 143 provided follow-up data. The proportion of MSS responders was 91%. Mean MSS decreased from 8.4 on day 0 (N=150) to 1.9 on day 15 (N=143). Patients reporting pain/discomfort and problems with usual activities decreased from 88.4% to 31.5% and from 43.2% to 1.4%, respectively, and mean VAS increased from 58.7 to 79.5. Intranasal corticosteroids were the most recommended and/or prescribed drugs. Total cost for an episode was 10,260 SEK (€1,102), of which 75% were indirect costs.
Conclusions:
With treatment dominated by intranasal corticosteroids, a high proportion of responders and good symptom relief were seen. Acute rhinosinusitis seems to cause a high burden on quality of life and also a high cost for society.
Similar content being viewed by others
Introduction
Symptoms consistent with acute rhinosinusitis are common clinical problems in primary care practice. In Europe, 1–2% of all patient visits to physicians in primary care are for suspected acute sinusitis.1 The high number of visits to physicians translates to a high burden on the healthcare system. In 2000 the direct costs in the United States were estimated to be nearly $6 billion.2,3
According to current European guidelines, the recommended treatment for mild acute rhinosinusitis is symptomatic, with nasal steroids advised in moderate cases.4,5 Antibiotics should only be added if severe symptoms are present (e.g. fever >38°C, severe pain). The evidence for the efficacy of decongestants in the treatment of acute rhinosinusitis in adults is poor, and decongestants only have a grade D recommendation.4,5
According to recent estimates, bacterial infection is present in ≤50% of patients with symptoms of acute rhinosinusitis6,7 and may be as low as 0.5–2%.8 Several studies have shown that antibiotics commonly used in Sweden are of limited value in the management of patients with mild to moderate acute rhinosinusitis.9–11 An increase in antibiotic resistance has also been observed among Streptococcus pneumoniae isolates globally, particularly to amoxicillin and other beta-lactam antibiotics.12 Despite this, antibiotics have previously been estimated to be prescribed to more than 90% of adult patients seen for acute rhinosinusitis in the UK.13 The proportion receiving antibiotics in Scandinavia has also been high.14 However, a recent questionnaire study performed in the Netherlands showed that current treatment patterns might have changed, as the antibiotic prescription frequency in that study was 20% and 34% for mild and moderate acute rhinosinusitis, respectively.15
Few studies investigating the impact of acute rhinosinusitis on disease-specific quality of life have been performed and generic quality of life data are limited.16 Furthermore, to our knowledge, the costs for acute rhinosinusitis have not previously been investigated in Scandinavia.
The purpose of this study was to investigate the treatment patterns in acute rhinosinusitis in primary care and to increase knowledge of the disease population, symptoms, quality of life and costs.
Methods
Study design and patients
This was a prospective non-interventional observational study in adults with acute rhinosinusitis performed at 11 Swedish primary care practices from November 2008 to December 2009. The study protocol was approved by the ethics committee in Stockholm, Sweden, and written informed consent was obtained from all patients before inclusion in the study.
The study protocol did not provide any restrictions for the treatment of acute rhinosinusitis. At the discretion of the investigator, medical treatment was prescribed in accordance with local standard practice, i.e. the chosen treatment by the investigator of each centre in this study for each patient with acute rhinosinusitis, as defined by the inclusion/exclusion criteria.
The primary objective and endpoint was to estimate the proportion of responders according to the Major Symptom Score (MSS) for patients with acute rhinosinusitis. A responder was predefined in the study protocol as a patient who improved in the MSS by at least 30% from day 0 to day 15 (preferably ±2 days). Secondary objectives and endpoints were: (1) to estimate the changes in MSS and individual item scores from day 0 to day 15 (preferably ±2 days); (2) to describe current drug treatment patterns; (3) to describe the health-related quality of life status associated with acute rhinosinusitis and related to the severity of the symptoms; and (4) to estimate the total costs related to episodes of acute rhinosinusitis and to the severity of the symptoms.
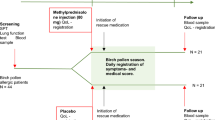
The study design is shown in Figure 1. Patients were recruited from the pool of patients attending the practice seeking medical treatment on an outpatient basis. Inclusion was based on duration of symptoms (≥7 but ≤28 days) and severity of acute rhinosinusitis (MSS ≥5 but ≤12; see Questionnaires and data collection forms, available as online appendices at www.thepcrj.org).10 Exclusion criteria included signs and symptoms suggestive of fulminant bacterial acute rhinosinusitis (fever ≥38.3°C or ≥38.5°C if a digital thermometer was not available, persistent severe unilateral facial or tooth pain, facial swelling, dental involvement or a worsening of symptoms after initial improvement).
On the inclusion day (day 0), questionnaires were completed by the patients, and demographic data, relevant medical history and recommended and/or prescribed medications were recorded by the investigator in a case report form. On day 15 (preferably ±2 days), a telephone follow-up was conducted by an independent medically-qualified person.
Questionnaires and data collection
Major Symptom Score (MSS)
The MSS17 was the sum of scores for the five major symptoms rhinorrhoea, postnasal drip, nasal congestion/stuffiness, sinus headache and facial pain/pressure/tenderness (see Appendix 1, available online at www.thepcrj.org). Each symptom was graded as 0 (none), 1 (mild), 2 (moderate) or 3 (severe). The patients were asked to grade symptoms related to acute rhinosinusitis that they had experienced during 12 h prior to the visit on day 0 and to the follow-up telephone call on day 15. The questionnaire was reviewed by the investigator on day 0
Quality of life
Quality of life was assessed by the EQ-5D™ (see Appendix 2 online).18 The patients were asked to grade their health status on days 0 and 15. Mobility, self-care, usual activities, pain/discomfort and anxiety/depression were graded as ‘none/no problems’, ‘moderate/some problems’ or ‘severe/extreme problems’. The patients also indicated their health status on a visual analogue scale (VAS) where 0 indicated worst conceivable status and 100 indicated best conceivable status.
Costs
The productivity, healthcare utilisation and treatment questionnaire completed on days 0 and 15 captured information on (1) outpatient visit and institutional care; (2) travel to the doctor; (3) employment status and absence due to illness; and (4) use of medications for acute rhinosinusitis (see Appendix 3 online). For the day 0 questionnaire, patients were asked to consider a recall period of 7 days. Thus the cost of ‘one episode’ of acute rhinosinusitis was all costs due to acute rhinosinusitis 7 days before the inclusion visit and 15 days after, a total of 22 days. To give productivity, transport and healthcare utilisation monetary values, unit costs were assigned. For cost of medication, as a principle, the cost of the smallest pack(s) corresponding to the reported use was used. An exception was made for painkillers (non-steroidal anti-inflammatory drugs, paracetamol, etc) as it was assumed that patients would have a supply of painkillers at home and thus only the daily cost of painkillers was used to estimate cost. Unit costs for healthcare resource use, transport and medication were based on published price lists. Unit cost for productivity was derived from the mean annual income. Direct cost was the sum of costs for outpatient visits and institutional care, travel to the doctor and use of medications. Transportation costs from patients' visits up until and on day 0 were included. Indirect cost was the sum of costs for loss of productivity as derived from answers to questions on employment status, reduced productivity and absence due to illness. Total cost was the sum of direct and indirect costs.
The questionnaire was completed by the patient after information about the disease by the investigator.
Statistical methods
All data were summarised by means of descriptive statistics and a 95% confidence interval (CI) for the proportion of responders was calculated. Pearson correlation was used to analyse EQ-5D™, VAS and total cost relationship to MSS.
For the presentation of descriptive statistics of EQ-5D™ and cost variables, the MSS symptom severity classes were defined as low (MSS 5–6), mid (MSS 7–9) and high (MSS 10–12).
Results
Studied population
A total of 150 patients were enrolled, of whom 143 provided data at follow-up (including six patients with MSS outside the prespecified MSS range).
Demographic data and main background characteristics are summarised in Table 1. The mean age was 45.1 years and 76.7% of the patients were female; 20.4% of the patients were current smokers and 2.7% were former smokers. The most common current or past medical history conditions were asthma (29.9%) and seasonal allergic rhinitis (26.5%). Sleep was impaired in approximately half of the patients due to the current episode of acute rhinosinusitis.
Primary endpoint
The proportion of responders (i.e. the proportion of patients in whom MSS improved by at least 30% from day 0 to day 15) was 90.9% (95% CI: 85.0% — 95.1%).
Secondary endpoints
Mean change in MSS from day 0 to day 15
Mean (SD) MSS decreased from 8.4 (2.3) on day 0 to 1.9 (2.2) on day 15. The mean (SD) change from baseline to day 15 in MSS was −6.4 (2.8). Ten patients showed no or limited change in MSS (0, 1 or −1). The mean (SD) change in individual MSS items were −1.5 (1.0) for sinus headache, −1.5 (1.0) for nasal congestion/ stuffiness, −1.3 (1.0) for facial pain/pressure/tenderness, −1.2 (0.9) for postnasal drip and −0.9 (1.1) for rhinorrhoea.
Recommended and/or prescribed medications and use of medications
Intranasal corticosteroids were the most recommended/prescribed medications (91%), followed by antibiotics (60%) and decongestant tablets (27%) (Table 2 and Figure 2).
EQ-5D™ dimensions and VAS
On day 0, pain/discomfort was reported by 88.4% of patients and problems with usual activities by 43.2%. Extreme pain/discomfort was reported by 10.9%, and 10.9% were also unable to perform their usual activities. Both dimensions showed improvement on day 15 when 31.5% of patients reported moderate/extreme pain and 1.4% reported having problems with usual activities. Self-care was the dimension least affected by acute rhinosinusitis (Figure 3).
Analysis of the EQ-5D™ data based on MSS symptom severity classes indicated that patients with higher MSS experienced extreme problems in the EQ-5D™ dimensions, mainly pain/discomfort and usual activities, to a greater extent than patients with lower MSS.
The mean (SD) VAS score improved from 58.7 (18.9) on day 0 to 79.5 (19.1) on day 15 (Table 3). The Pearson correlation coefficient between VAS and MSS on day 15 was −0.37. The Pearson correlation coefficient between VAS and MSS for the individual item scores on day 15 was −0.28 for facial pain or tenderness on palpation, −0.32 for sinus headache and −0.37 for nasal congestion/stuffiness.
Costs (direct and indirect costs)
The mean total cost for one episode of acute rhinosinusitis was 10,260 SEK (€1,102 at May 2011, 1 SEK = €0.11), of which mean direct costs were 2,478 SEK (€266) (Table 4). The inter-individual variability in indirect costs was large (minimum 0 and maximum 44,258 SEK (€4,752)).
There was no trend in direct, indirect or total costs based on MSS symptom severity classes. Mean total costs ranged from 9,663 SEK (€1,037) for low severity (MSS 5–6) to 10,154 SEK (€1,090) for high severity (MSS 10–12).
The Pearson correlation coefficients between direct, indirect and total costs and MSS were 0.00, 0.11 and 0.11, respectively, on day 0, and 0.18, 0.11 and 0.13, respectively, on day 15.
Discussion
Main findings
In this observational study in primary care, nine out of 10 adult patients with acute rhinosinusitis showed significant improvement in symptoms 15 days after a visit to a primary care physician where patients were treated according to local practice, and 91% of patients were recommended and/or prescribed intranasal corticosteroids.
As the diagnosis ‘acute rhinosinusitis’ does not exist in the official Swedish registry of diagnoses, based on WHO International Classification of Diseases (ICD-10), it was not possible to perform a retrospective or prospective registry study in this case. We thus needed to perform a prospective observational study to collect data.
The study design was therefore based on the EP3OS 2007 recommendations for study definitions of acute rhinosinusitis and outcomes in primary care.4,5 The inclusion criteria were based on data from an earlier clinical study,10 international guidelines4,5 and Swedish clinical practice. This approach tried to exclude ‘common colds’ by excluding patients with a symptom duration shorter than 7 days and an MSS <5, as well as excluding severe acute rhinosinusitis by not including patients with MSS >12 and by applying the exclusion criteria.
The results from the analyses of the primary endpoint showed a very high proportion of responders, with about 90% of patients demonstrating a clinically relevant improvement during the time period investigated. The results further showed that there was good symptom relief within the studied time period, as mean MSS decreased from 8.4 at baseline to 1.9 on day 15. This was a greater improvement than seen in the randomised placebo-controlled clinical study by Meltzer et al. in which MSS was used as a primary endpoint to compare mometasone furoate nasal spray with amoxicillin and placebo.10 However, it should be noted that our study is not easily comparable with the study by Meltzer et al. as it was observational and was not designed to compare efficacy and safety of treatments for acute rhinosinusitis. Furthermore, MSS was used in a different way in our study from that in the study by Meltzer et al.
The treatments prescribed and used during the study period consisted mainly of intranasal corticosteroids and/or antibiotics. The finding of the surprisingly high use of intranasal steroids is an indication of a change in the clinical treatment of acute rhinosinusitis in primary care, consistent with the EP3OS evidence-based guidelines.4,5
The slight decrease in the number of patients using nasal corticosteroids and the corresponding increase in patients using antibiotics during the 15 days of study remains to be explained. One theory could be that some patients did not respond to initial nasal steroid treatment and were switched to antibiotics.
Interpretation of findings in relation to previously published work
A comparison of the results from the current study with a recently published French survey of 397 general practitioners (GPs), summarising data from 1,585 patients, showed that the use of intranasal steroids was higher (91% vs. 38.7%) in our study and the use of oral antibiotics was lower (60% vs. 86.5%).19 However, the study populations differed in characteristics as the French questionnaire survey was based on physician diagnosis of acute maxillary sinusitis with signs of a bacterial infection. A recent study performed in the Netherlands using questionnaires sent to GPs showed that oral antibiotics were prescribed by approximately one-third of the practitioners for mild and moderate acute rhinosinusitis.15 In the current study, we found that antibiotic treatment was common, despite national programmes to reduce its use.20 The most recent Cochrane update emphasised that antibiotics have a limited treatment effect in acute rhinosinusitis and stated that most cases will resolve without antibiotics within 2 weeks.21 Our interpretation of the data is that, in contrast to medical evidence and recent guidelines, there still seems to be a general view among GPs that mild to moderate acute rhinosinusitis is the result of a bacterial infection.
In the Dutch study, decongestants were the most commonly prescribed treatments for both mild (91%) and moderate (83%) acute rhinosinusitis and were the first choice treatment in both cases.15 The use of decongestants was approximately three times more common than in our study, which is interesting as the evidence level is low for the efficacy of decongestants in mild to moderate disease.4,5 However, only one-third of the GPs in the Dutch study considered prescribing intranasal corticosteroids in mild or moderate acute rhinosinusitis.
The quality of life measure chosen by us was a generic and short validated method, the EQ-5D™. As this is a non-interventional study, any ‘intervention’ (including time for filling out questionnaires) outside common clinical practice, taking an unreasonably long time for the patient (or the primary care centre) could have jeopardised the approval by the Ethics Committee.
The data suggest a high burden of acute rhinosinusitis on quality of life parameters, an area that has not so far been well studied. At baseline, pain/discomfort was reported by 88.4% of the patients and problems with usual activities by 43.2%. Both dimensions showed improvement on day 15. Investigation of the correlation between MSS and quality of life showed that increased MSS to a large extent gave worse problems in the EQ-5D™, mostly pain/discomfort and usual activities. The mean health status measured using the VAS score improved from day 0 to day 15 and there was some correlation between EQ-5D™, VAS and MSS on day 15. The data are in line with the results from a randomised placebo-controlled study by Bachert and Meltzer which demonstrated significant improvement in disease-specific quality of life among effectively treated patients with acute rhinosinusitis.16 However, the SNOT-20 questionnaire used in that study was originally not developed for acute rhinosinusitis. Quality of life deserves further investigation and is a recommended assessment to be included in clinical studies of acute rhinosinusitis.22
The mean total cost for an episode of acute mild to moderate rhinosinusitis in patients seeking primary care was 10,260 SEK (approximately €1,102 at May 2011). The direct costs (medications, visits to the physician) constituted only about 25% of the total costs, indicating that the main costs for acute rhinosinusitis are due to loss in productivity (indirect costs). There was a large difference between mean and median numbers in the cost analysis, and a few patients reported very high costs. In cost of illness studies it is not uncommon to find large variability in patient level cost estimates.23,24 When the cost was correlated to the MSS, some correlation between direct, indirect and total costs and MSS on day 15 could be shown. There was also some correlation for indirect and total costs with MSS on day 0.
This is the first observational study in Scandinavia to address costs for acute rhinosinusitis in primary care. A recent Swedish questionnaire study of allergic rhinitis and the common cold used a similar method to estimate productivity loss and found a mean productivity loss of €653 per worker per year, adding up to an indirect cost of €2.7 billion for society as a whole.25 Although acute rhinosinusitis is arguably less prevalent than allergic rhinitis and the common cold, the high cost per episode suggests that acute rhinosinusitis has a considerable financial impact on society.
Limitations and strengths of this study
Weaknesses of the study are the recognised problems of observational studies as well as, in our case, a small population size and the use of a non-validated productivity, healthcare utilisation and treatment questionnaire. Having a pharmaceutical company as a sponsor of the study could potentially influence the treatment choices, even though local Swedish guidelines and European legislation on conduct of non-interventional studies were followed.
On the other hand, the strengths of our study are the primary care ‘real-life’ setting and the prospective design with independent follow-up. Furthermore, we used validated questions on quality of life (EQ-5D™) and symptom score (MSS).
Conclusions
In conclusion, with a pharmacological treatment pattern dominated by intranasal corticosteroids, a high proportion of responders and good symptom relief were seen within the study time period. In addition, our data suggest a high burden of acute rhinosinusitis on quality of life and also a high cost for society, two areas that have not been well studied in Europe.
References
Lindbaek M . Acute sinusitis: guide to selection of antibacterial therapy. Drugs 2004;64(8):805–19. http://dx.doi.org/10.2165/00003495-200464080-00002
Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol 2005;116(6 Suppl):S13–S47. http://dx.doi.org/10.1016/j.jaci.2005.09.048
Sande MA and Gwaltney JM . Acute community-acquired bacterial sinusitis: continuing challenges and current management. Clin Infect Dis 2004;39(Suppl 13):S151–8. http://dx.doi.org/10.1086/421353.
Fokkens W, Lund V, Mullol J . European position paper on rhinosinusitis and nasal polyps. Rhinol Suppl 2007;20:1–136.
Thomas M, Yawn BP, Price D, Lund V, Mullol J, Fokkens W . EPOS Primary Care Guidelines: European position paper on the primary care diagnosis and management of rhinosinusitis and nasal polyps 2007 — a summary. Prim Care Respir J 2008;17(2):79–89. http://dx.doi.org/10.3132/pcrj.2008.00029.
Hickner JM . Acute rhinosinusitis: a diagnostic and therapeutic challenge. J Fam Pract 2001;50(1):38–40.
Piccirillo JF . Clinical practice. Acute bacterial sinusitis. N Engl J Med 2004;351(9):902–10. http://dx.doi.org/10.1056/NEJMcp035553.
Anon JB, Jacobs MR, Poole MD, et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004;130(1 Suppl):1–45. http://dx.doi.org/10.1016/j.otohns.2003.12.003
Stalman W, van Essen GA, van der Graaf Y, de Melker RA . The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice? A placebo-controlled double-blind randomized doxycycline trial. Br J Gen Pract 1997;47(425):794–9.
Meltzer EO, Bachert C, Staudinger H . Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol 2005;116(6):1289–95. http://dx.doi.org/10.1016/j.jaci.2005.08.044.
Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet 2008;371(9616):908–14. http://dx.doi.org/10.1016/S0140-6736(08)60416-X.
Felmingham D, Reinert RR, Hirakata Y, Rodloff A . Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and comparative in vitro activity of the ketolide, telithromycin. J Antimicrob Chemother 2002;50(Suppl S1):25–37.
Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M . Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br J Gen Pract 2005;55(517):603–08.
Berg O, Carenfelt C, Kronvall G . Bacteriology of maxillary sinusitis in relation to character of inflammation and prior treatment. Scand J Infect Dis 1988;20(5):511–16. http://dx.doi.org/10.3109/00365548809032499
Hoffmans R, Schermer T, van Weel C, Fokkens W . Management of rhinosinusitis in Dutch general practice. Prim Care Respir J 2011;20(1):64–70. http://dx.doi.org/10.4104/pcrj.2010.00064.
Bachert C, Meltzer EO . Effect of mometasone furoate nasal spray on quality of life of patients with acute rhinosinusitis. Rhinology 2007;45(3):190–6.
Revicki D, Margolis M, Thompson C, Meltzer E, Sandor D, Shaw J . Major Symptom Score Utility Index for patients with acute rhinosinusitis. Am J Rhinol Allergy 2011;25(3):99–106. http://dx.doi.org/10.2500/ajra.2011.11.3575.
Rabin R, de Charro F . EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33(5):337–43. http://www.euroqol.org. http://dx.doi.org/10.3109/07853890109002087
Klossek JM, Mesbah K . Presentation and treatment of acute maxillary sinusitis in general practice: a French observational study. Rhinology 2011;49(1):84–9.
Mölstad S, Erntell M, Hanberger H, et al. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis 2008;8(2):125–32. http://dx.doi.org/10.1016/S1473-3099(08)70017-3.
Ahovuo-Saloranta A, Borisenko OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2008;(2):CD000243. http://dx.doi.org/10.1002/14651858.CD000243.pub2.
Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 2004;114(6 suppl):155–212. http://dx.doi.org/10.1016/j.jaci.2004.09.029.
Jansson SA, Ronmark E, Forsberg B, Löfgren C, Lindberg A, Lundbäck B . The economic consequences of asthma among adults in Sweden. Respir Med 2007;101(11):2263–70. http://dx.doi.org/10.1016/j.rmed.2007.06.029.
Gendo K, Sullivan SD, Lozano P, Finkelstein JA, Fuhlbrigge A, Weiss KB . Resource costs for asthma-related care among pediatric patients in managed care. Ann Allergy Asthma Immunol 2003;91(3):251–7. http://dx.doi.org/10.1016/S1081-1206(10)63526-0.
Hellgren J, Cervin A, Nordling S, Bergman A, Cardell LO . Allergic rhinitis and the common cold — high cost to society. Allergy 2010;65(6):776–83. http://dx.doi.org/10.1111/j.1398-9995.2009.02269.x.
Acknowledgements
Handling editor Osman Mohammed Yusuf
Statistical review Gopal Netuveli
Funding
MSD (Sweden) AB funded this study, as well as the editorial support by Hanna Liedman.
The authors thank Patrick Svarvar (Merck) for input to the study design, Catarina Jansson-Blixt (TFS Sweden) for statistical support, Sari von Reedtz (MSD) for supporting the study report, Johanna Andlin (MSD) for supporting the study protocol and Hanna Liedman (TFS Sweden) for editorial support. The authors also thank all the investigators in the study; Rickard Ekesbo, Dalby; Ines Vinge, Lindingö; Bo Polhem, Uddevalla; Ingrid Johansson, Fjugesta; Tomas Lindskog, Växjö; Christer Dahlqvist, Limhamn; Per Hellke, Göteborg; Kjell Larsson, Arvidsjaur; Anne-Sofie Lindberg, Bro; Maria Papachristou Örebro; and Luisa Escuder, Bromma, all in Sweden.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this paper.
Corresponding author
Ethics declarations
Competing interests
BS and P Odebäck have received honoraria for educational activities from MSD, GSK and AstraZeneca. PS has received grants for studies and honoraria for educational activities from MSD, GSK, AstraZeneca and Novartis. P Olsson was employed by MSD during the conduct of the study and is currently employed by Boehringer-Ingelheim. JL is an employee of MSD and owns stock options and shares in Merck & Co. BS is an Associate Editor of the PCRJ, but was not involved in the editorial review of, nor the decision to publish, this article.
Appendices
Appendix 1. MSS questionnaire



Appendix 2.



Appendix 3. Productivity, healthcare utilisation and treatment questionnaire




Rights and permissions
About this article
Cite this article
Stjärne, P., Odebäck, P., Ställberg, B. et al. High costs and burden of illness in acute rhinosinusitis: real-life treatment patterns and outcomes in Swedish primary care. Prim Care Respir J 21, 174–179 (2012). https://doi.org/10.4104/pcrj.2012.00011
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4104/pcrj.2012.00011
This article is cited by
-
Type 2 and Non-type 2 Inflammation in the Upper Airways: Cellular and Molecular Alterations in Olfactory Neuroepithelium Cell Populations
Current Allergy and Asthma Reports (2024)
-
A EUFOREA comment on a lost comorbidity of asthma
Allergy, Asthma & Clinical Immunology (2023)
-
Effects of nasal septum deviation and concha bullosa surgery on the frequency and financial burden of acute rhinosinusitis
Irish Journal of Medical Science (1971 -) (2023)
-
Common Cold and Acute Rhinosinusitis: Up-to-Date Management in 2020
Current Allergy and Asthma Reports (2020)