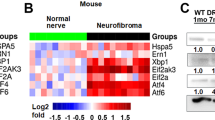
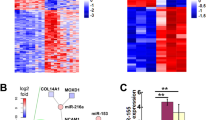
Abstract
Loss of function mutations in the neurofibromatosis Type 2 (NF2) gene, coding for a tumour suppressor, Merlin, cause multiple tumours of the nervous system such as schwannomas, meningiomas and ependymomas. These tumours may occur sporadically or as part of the hereditary condition neurofibromatosis Type 2 (NF2). Current treatment is confined to (radio) surgery and no targeted drug therapies exist. NF2 mutations and/or Merlin inactivation are also seen in other cancers including some mesothelioma, breast cancer, colorectal carcinoma, melanoma and glioblastoma. To study the relationship between Merlin deficiency and tumourigenesis, we have developed an in vitro model comprising human primary schwannoma cells, the most common Merlin-deficient tumour and the hallmark for NF2. Using this model, we show increased expression of cellular prion protein (PrPC) in schwannoma cells and tissues. In addition, a strong overexpression of PrPC is observed in human Merlin-deficient mesothelioma cell line TRA and in human Merlin-deficient meningiomas. PrPC contributes to increased proliferation, cell-matrix adhesion and survival in schwannoma cells acting via 37/67 kDa non-integrin laminin receptor (LR/37/67 kDa) and downstream ERK1/2, PI3K/AKT and FAK signalling pathways. PrPC protein is also strongly released from schwannoma cells via exosomes and as a free peptide suggesting that it may act in an autocrine and/or paracrine manner. We suggest that PrPC and its interactor, LR/37/67 kDa, could be potential therapeutic targets for schwannomas and other Merlin-deficient tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hanemann CO . Magic but treatable? Tumours due to loss of merlin. Brain 2008; 131 (Pt 3): 606–615.
Petrilli AM, Fernandez-Valle C . Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016; 35: 537–548.
Blakeley JO, Evans DG, Adler J, Brackmann D, Chen R, Ferner RE et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A 2012; 158A: 24–41.
Rosenbaum C, Kluwe L, Mautner VF, Friedrich RE, Mueller HW, Hanemann CO . Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis 1998; 5: 55–64.
Ammoun S, Provenzano L, Zhou L, Barczyk M, Evans K, Hilton DA et al. Axl/Gas6/NFkappaB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene 2014; 33: 336–346.
Ammoun S, Schmid MC, Zhou L, Hilton DA, Barczyk M, Hanemann CO . The p53/mouse double minute 2 homolog complex deregulation in merlin-deficient tumours. Mol Oncol 2015; 9: 236–248.
Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 2010; 140: 477–490.
Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO . Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia 2011; 13: 1101–1112.
Hanemann CO, Bartelt-Kirbach B, Diebold R, Kampchen K, Langmesser S, Utermark T . Differential gene expression between human schwannoma and control Schwann cells. Neuropathol Appl Neurobiol 2006; 32: 605–614.
Westergard L, Christensen HM, Harris DA . The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta 2007; 1772: 629–644.
Ford MJ, Burton LJ, Morris RJ, Hall SM . Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 2002; 113: 177–192.
Steele AD, Emsley JG, Ozdinler PH, Lindquist S, Macklis JD . Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci USA 2006; 103: 3416–3421.
Bremer J, Baumann F, Tiberi C, Wessig C, Fischer H, Schwarz P et al. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci 2010; 13: 310–318.
Stella R, Massimino ML, Sandri M, Sorgato MC, Bertoli A . Cellular prion protein promotes regeneration of adult muscle tissue. Mol Cell Biol 2010; 30: 4864–4876.
Santos TG, Lopes MH, Martins VR . Targeting prion protein interactions in cancer. Prion 2015; 9: 165–173.
Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR . Physiology of the prion protein. Physiol Rev 2008; 88: 673–728.
Zeng L, Zou W, Wang G . Cellular prion protein (PrP(C)) and its role in stress responses. Int J Clin Exp Med 2015; 8: 8042–8050.
Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO . Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res 2008; 68: 5236–5245.
Ammoun S, Schmid MC, Zhou L, Ristic N, Ercolano E, Hilton DA et al. Insulin-like growth factor-binding protein-1 (IGFBP-1) regulates human schwannoma proliferation, adhesion and survival. Oncogene 2011; 31: 1710–1722.
Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M et al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA 2004; 101: 9683–9688.
Guillot-Sestier MV, Sunyach C, Druon C, Scarzello S, Checler F . The alpha-secretase-derived N-terminal product of cellular prion, N1, displays neuroprotective function in vitro and in vivo. J Biol Chem 2009; 284: 35973–35986.
Zuber C, Knackmuss S, Rey C, Reusch U, Rottgen P, Frohlich T et al. Single chain Fv antibodies directed against the 37 kDa/67 kDa laminin receptor as therapeutic tools in prion diseases. Mol Immunol 2008; 45: 144–151.
Sarnataro D, Pepe A, Altamura G, De Simone I, Pesapane A, Nitsch L et al. The 37/67 kDa laminin receptor (LR) inhibitor, NSC47924, affects 37/67 kDa LR cell surface localization and interaction with the cellular prion protein. Sci Rep 2016; 6: 24457.
Klyubin I, Nicoll AJ, Khalili-Shirazi A, Farmer M, Canning S, Mably A et al. Peripheral administration of a humanized anti-PrP antibody blocks Alzheimer's disease Abeta synaptotoxicity. J Neurosci 2014; 34: 6140–6145.
Lin Y, Bai L, Chen W, Xu S . The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets 2010; 14: 45–55.
Yap YH, Say YH . Resistance against apoptosis by the cellular prion protein is dependent on its glycosylation status in oral HSC-2 and colon LS 174T cancer cells. Cancer Lett 2011; 306: 111–119.
Ma J, Lindquist S . Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. Proc Natl Acad Sci USA 2001; 98: 14955–14960.
Deguen B, Goutebroze L, Giovannini M, Boisson C, van der NR, Jaurand MC et al. Heterogeneity of mesothelioma cell lines as defined by altered genomic structure and expression of the NF2 gene. Int J Cancer 1998; 77: 554–560.
Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol 2005; 25: 2384–2394.
Gerber MA, Bahr SM, Gutmann DH . Protein 4.1B/differentially expressed in adenocarcinoma of the lung-1 functions as a growth suppressor in meningioma cells by activating Rac1-dependent c-Jun-NH(2)-kinase signaling. Cancer Res 2006; 66: 5295–5303.
Nunes F, Shen Y, Niida Y, Beauchamp R, Stemmer-Rachamimov AO, Ramesh V et al. Inactivation patterns of NF2 and DAL-1/4.1B (EPB41L3) in sporadic meningioma. Cancer Genet Cytogenet 2005; 162: 135–139.
Wozniak K, Piaskowski S, Gresner SM, Golanska E, Bieniek E, Bigoszewska K et al. BCR expression is decreased in meningiomas showing loss of heterozygosity of 22q within a new minimal deletion region. Cancer Genet Cytogenet 2008; 183: 14–20.
van den Munckhof P, Christiaans I, Kenter SB, Baas F, Hulsebos TJ . Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. Neurogenetics 2012; 13: 1–7.
Yuzawa S, Nishihara H, Tanaka S . Genetic landscape of meningioma. Brain Tumor Pathol 2016; 33: 237–247.
Li W, Giancotti FG . Merlin's tumor suppression linked to inhibition of the E3 ubiquitin ligase CRL4 (DCAF1). Cell Cycle 2010; 9: 4433–4436.
Zhou L, Hanemann CO . Merlin, a multi-suppressor from cell membrane to the nucleus. FEBS Lett 2012; 586: 1403–1408.
Parkin ET, Watt NT, Turner AJ, Hooper NM . Dual mechanisms for shedding of the cellular prion protein. J Biol Chem 2004; 279: 11170–11178.
Altmeppen HC, Prox J, Puig B, Kluth MA, Bernreuther C, Thurm D et al. Lack of a-disintegrin-and-metalloproteinase ADAM10 leads to intracellular accumulation and loss of shedding of the cellular prion protein in vivo. Mol Neurodegener 2011; 6: 36.
Utermark T, Kaempchen K, Hanemann CO . Pathological adhesion of primary human schwannoma cells is dependent on altered expression of integrins. Brain Pathol 2003; 13: 352–363.
Weise J, Doeppner TR, Muller T, Wrede A, Schulz-Schaeffer W, Zerr I et al. Overexpression of cellular prion protein alters postischemic Erk1/2 phosphorylation but not Akt phosphorylation and protects against focal cerebral ischemia. Restor Neurol Neurosci 2008; 26: 57–64.
Arsenault RJ, Li Y, Potter A, Griebel PJ, Kusalik A, Napper S . Induction of ligand-specific PrP (C) signaling in human neuronal cells. Prion 2012; 6: 477–488.
Zhou XM, Xu GX, Zhao DM . In vitro effect of prion peptide PrP 106-126 on mouse macrophages: possible role of macrophages in transport and proliferation for prion protein. Microb Pathog 2008; 44: 129–134.
Parkinson DB, Bhaskaran A, rthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC et al. c-Jun is a negative regulator of myelination. J Cell Biol 2008; 181: 625–637.
Gu Y, Fujioka H, Mishra RS, Li R, Singh N . Prion peptide 106-126 modulates the aggregation of cellular prion protein and induces the synthesis of potentially neurotoxic transmembrane PrP. J Biol Chem 2002; 277: 2275–2286.
Robb VA, Gerber MA, Hart-Mahon EK, Gutmann DH . Membrane localization of the U2 domain of Protein 4.1B is necessary and sufficient for meningioma growth suppression. Oncogene 2005; 24: 1946–1957.
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013; 339: 1077–1080.
Bourteele S, Oesterle K, Weinzierl AO, Paxian S, Riemann M, Schmid RM et al. Alteration of NF-kappaB activity leads to mitochondrial apoptosis after infection with pathological prion protein. Cell Microbiol 2007; 9: 2202–2217.
Bacot SM, Lenz P, Frazier-Jessen MR, Feldman GM . Activation by prion peptide PrP106-126 induces a NF-kappaB-driven proinflammatory response in human monocyte-derived dendritic cells. J Leukoc Biol 2003; 74: 118–125.
Guo BB, Bellingham SA, Hill AF . Stimulating the release of exosomes increases the intercellular transfer of prions. J Biol Chem 2016; 291: 5128–5137.
Robertson C, Booth SA, Beniac DR, Coulthart MB, Booth TF, McNicol A . Cellular prion protein is released on exosomes from activated platelets. Blood 2006; 107: 3907–3911.
Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 2006; 31: 642–648.
Alais S, Simoes S, Baas D, Lehmann S, Raposo G, Darlix JL et al. Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles. Biol Cell 2008; 100: 603–615.
Lopes JP, Oliveira CR, Agostinho P . Cdk5 acts as a mediator of neuronal cell cycle re-entry triggered by amyloid-beta and prion peptides. Cell Cycle 2009; 8: 97–104.
Satoh J, Kuroda Y, Katamine S . Gene expression profile in prion protein-deficient fibroblasts in culture. Am J Pathol 2000; 157: 59–68.
Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J 2007; 21: 2247–2256.
Ammoun S, Ristic N, Matthies C, Hilton DA, Hanemann CO . Targeting ERK1/2 activation and proliferation in human primary schwannoma cells with MEK1/2 inhibitor AZD6244. Neurobiol Dis 2010; 37: 141–146.
Godsave SF, Peters PJ, Wille H . Subcellular distribution of the prion protein in sickness and in health. Virus Res 2015; 207: 136–145.
Singh N, Singh A, Das D, Mohan ML . Redox control of prion and disease pathogenesis. Antioxid Redox Signal 2010; 12: 1271–1294.
Gauczynski S, Peyrin JM, Haik S, Leucht C, Hundt C, Rieger R et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J 2001; 20: 5863–5875.
Watts JC, Westaway D . The prion protein family: diversity, rivalry, and dysfunction. Biochim Biophys Acta 2007; 1772: 654–672.
Loubet D, Dakowski C, Pietri M, Pradines E, Bernard S, Callebert J et al. Neuritogenesis: the prion protein controls beta1 integrin signaling activity. FASEB J 2012; 26: 678–690.
Carimalo J, Cronier S, Petit G, Peyrin JM, Boukhtouche F, Arbez N et al. Activation of the JNK-c-Jun pathway during the early phase of neuronal apoptosis induced by PrP106-126 and prion infection. Eur J Neurosci 2005; 21: 2311–2319.
Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R et al. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol 2004; 164: 385–394.
Shivane A, Parkinson DB, Ammoun S, Hanemann CO . Expression of c-Jun and Sox-2 in human schwannomas and traumatic neuromas. Histopathology 2013; 62: 651–656.
Pflanzner T, Petsch B, Andre-Dohmen B, Muller-Schiffmann A, Tschickardt S, Weggen S et al. Cellular prion protein participates in amyloid-beta transcytosis across the blood-brain barrier. J Cereb Blood Flow Metab 2012; 32: 628–632.
Rosenbaum C, Kluwe L, Mautner VF, Friedrich RE, Muller HW, Hanemann CO . Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis 1998; 5: 55–64.
Kimata A, Nakagawa H, Ohyama R, Fukuuchi T, Ohta S, Doh-ura K et al. New series of antiprion compounds: pyrazolone derivatives have the potent activity of inhibiting protease-resistant prion protein accumulation. J Med Chem 2007; 50: 5053–5056.
Flaiz C, Ammoun S, Biebl A, Hanemann CO . Altered adhesive structures and their relation to RhoGTPase activation in merlin-deficient Schwannoma. Brain Pathol 2009; 19: 27–38.
Hilton DA, Shivane A, Kirk L, Bassiri K, Enki DG, Hanemann CO . Activation of multiple growth factor signalling pathways is frequent in meningiomas. Neuropathology 2016; 36: 250–261.
Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA 2005; 102: 9182–9187.
Acknowledgements
We thank Mario Salmona for mock PrP peptide and Joseph Testa for Merlin adenovirus. This work was supported by The Laura Crane Youth Cancer Trust and Brain Tumour Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Provenzano, L., Ryan, Y., Hilton, D. et al. Cellular prion protein (PrPC) in the development of Merlin-deficient tumours. Oncogene 36, 6132–6142 (2017). https://doi.org/10.1038/onc.2017.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.200
This article is cited by
-
Anchorless risk or released benefit? An updated view on the ADAM10-mediated shedding of the prion protein
Cell and Tissue Research (2023)
-
Emerging roles of the cellular prion protein (PrPC) and 37/67 kDa laminin receptor (RPSA) interaction in cancer biology
Cellular and Molecular Life Sciences (2023)
-
The multiple functions of PrPC in physiological, cancer, and neurodegenerative contexts
Journal of Molecular Medicine (2022)
-
The cellular prion protein is a stress protein secreted by renal tubular cells and a urinary marker of kidney injury
Cell Death & Disease (2020)
-
Structural and mechanistic aspects influencing the ADAM10-mediated shedding of the prion protein
Molecular Neurodegeneration (2018)