Abstract
With very similar 3D structures, the widely expressed β-arrestin isoforms 1 and 2 play at times identical, distinct or even opposing roles in regulating various aspects of G protein-coupled receptors (GPCR) expression and signalling. Recent evidence recognizes the β-arrestin system as a key regulator of not only GPCRs, but also receptor tyrosine kinases, including the highly cancer relevant insulin-like growth factor type 1 receptor (IGF-1R). Binding of β-arrestin1 to IGF-1R leads to ligand-dependent degradation of the receptor and generates additional MAPK/ERK signalling, protecting cancer cells against anti-IGF-1R therapy. Because the interplay between β-arrestin isoforms governs the biological effects for most GPCRs, as yet unexplored for the IGF-1R, we sought to investigate specifically the regulatory roles of the β-arrestin2 isoform on expression and function of the IGF-1R. Results from controlled expression of either β-arrestin isoform demonstrate that β-arrestin2 acts in an opposite manner to β-arrestin1 by promoting degradation of an unstimulated IGF-1R, but protecting the receptor against agonist-induced degradation. Although both isoforms co-immunoprecipitate with IGF-1R, the ligand-occupied receptor has greater affinity for β-arrestin1; this association lasts longer, sustains MAPK/ERK signalling and mitigates p53 activation. Conversely, β-arrestin2 has greater affinity for the ligand-unoccupied receptor; this interaction is transient, triggers receptor ubiquitination and degradation without signalling activation, and leads to a lack of responsiveness to IGF-1, cell cycle arrest and decreased viability of cancer cells. This study reveals contrasting abilities of IGF-1R to interact with each β-arrestin isoform, depending on the presence of the ligand and demonstrates the antagonism between the two β-arrestin isoforms in controlling IGF-1R expression and function, which could be developed into a practical anti-IGF-1R strategy for cancer therapy.
Similar content being viewed by others
Introduction
The arrestin system plays a well-established role in regulating the spatio-temporal characteristics of signalling downstream of GPCRs. Of the four mammalian arrestin isoforms, two are found only in photoreceptor cells of the eye (arrestin1 and arrestin4, the visual arrestins), whereas the other two non-visual arrestins, termed arrestin2 and -3, more commonly known as β-arrestin1 and 2 (β-arr1 and 2 throughout), are ubiquitously expressed.1 β-arrestin recruitment and binding to the ligand-stimulated receptor is essential for desensitization, sequestration, recycling and downregulation of most GPCRs.2, 3 The amino-acid sequences of the two non-visual isoforms are 78% identical, diverging most in the C-terminal regions.4 Knockout mice models show that β-arrs can at least partially functionally substitute for each other as the single knockout phenotype is viable, whereas the double-knockout phenotype is embryonic lethal.5, 6, 7 Nonetheless, in vitro and in vivo studies do not support completely redundant roles for all β-arr-mediated functions: internalization of some GPCRs like the angiotensin II type 1A receptor is mediated in the same manner by both β-arr isoforms,8, 9 whereas for other receptors only one isoform is involved (for example, β-arr2 for β2-adrenergic receptor).10, 11 The corollary of these data is that despite a very similar 3D structure, the two β-arr isoforms have distinct roles in regulating functional characteristics of GPCR signalling.4, 12, 13
Intriguingly, accumulating evidence recognizes the β-arr system as a key regulator of not only GPCRs, but another major class of cell surface receptors; receptor tyrosine kinases, including the insulin-like growth factor type 1 receptor (IGF-1R), epidermal growth factor receptor and insulin receptor.14 Of these, the IGF-1R has gained much attention for its central role in cancer cell growth and survival. Shown to be essential for malignant transformation by many classical oncogenes,15, 16 its roles include not only proliferation and cell survival, but also key elements of the metastatic phenotype such as anchorage-independent growth, migration, invasion and tumour neovascularization.17, 18, 19, 20 There is, therefore, justifiably a focus for IGF-1R therapeutic antagonism, yet despite promising preclinical results, its targeting has proven more complex in clinical settings. One possible explanation is that all anti-IGF-1R therapeutic strategies so far have been designed mainly based on the tyrosine kinase receptor paradigm of the IGF-1R,21, 22 while overlooking the kinase-independent capabilities of the receptor.23, 24, 25, 26 Especially relevant, recent studies demonstrated that Mdm2-mediated ubiquitination of the IGF-1R, orchestrated by the G protein-coupled receptor kinases (GRK)/β-arr system,22, 24, 27, 28 is a central mechanism controlling the response to anti-IGF-1R-targeted therapy.29, 30, 31
It is now clear that in much the same way as with the GPCRs, ligand-induced conformational changes within the IGF-1R promotes recruitment of cytoplasmic β-arrs to the receptor GRK-phosphorylated serine sites.32, 33 Through this interaction, while initiating removal of the receptor from the cell surface (internalization), β-arrs also act as signalling mediators, connecting the receptor with downstream effectors such as components of the MAPK pathway.27, 34, 35, 36
Although the key role of β-arr1 in controlling signalling downstream of the IGF-1R is well recognized,28 there is very little information surrounding the involvement of β-arr2 in these processes. We therefore sought to investigate specifically the regulatory roles of the β-arr2 isoform on IGF-1R expression and function.
Results
Effect of β-arrestin2 modulation on IGF-1R ligand-dependent degradation
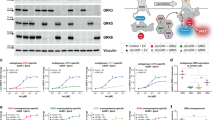
A growing body of evidence demonstrates that β-arr1 recruitment to an agonist-stimulated IGF-1R leads to receptor degradation,28, 29, 32, 37, 38 whereas the role of β-arr2 in this process has not yet been studied in detail. Thus, we initially compared the kinetic characteristics of ligand-induced IGF-1R degradation in mouse embryonic fibroblast (MEF) cells derived from wild-type mice or from their littermates that are knockout for either β-arr isoform.5, 6 Following western blot (WB) confirmation of β-arr expression levels in all cell lines (Figure 1a, left panel), receptor degradation was monitored by WB detection of IGF-1R levels in serum-starved cells stimulated with IGF-1 for up to 24 h (Figure 1a). The IGF-1R degradation rate is increased in the cells expressing only β-arr1 (β2KO) and decreased in MEF with only β-arr2 (β1KO) as compared to the cells expressing both isoforms (WT). These trends were confirmed by densitometry quantification of multiple experiments (Figure 1a, graph).
Effect of β-arrestin2 on IGF-1R ligand-dependent degradation. (a–c) MEFs knockout for either β-arr isoform (a) or MEF WT (b) and HEK293T (HEK) (c) transfected with isoform-specific β-arr-encoding plasmids (+β1/+β2), siRNAs (-β1/-β2) or respective controls (M) were lysed and endogenous (a) and post-transfection (b, c) levels of β-arrs were verified by WB, using GAPDH as a loading control (left panels). Cells transfected as indicated were serum starved and then stimulated with IGF-1 (50 ng/ml) for 0, 12 and 24 h and lysates were analysed by WB for IGF-1R, and GAPDH as a loading control (middle panels). IGF-1R signals were quantified by densitometry, normalized to GAPDH and expressed as a percentage of the IGF-1R in unstimulated cells (right panels). Data correspond to the mean±s.e.m. from three independent experiments. Statistical analysis (two-tailed t-test): IGF-1-induced degradation rate following β-arr modulation (in KO, +, −) compared with their respective WT or M (empty vector/non-target siRNA) controls, *P<0.05, **P<0.01.
These findings were confirmed using WT MEF cells transiently transfected with either β-arr1 or two selective siRNA, or β-arr-expressing plasmids, to reveal their functions by minimizing or increasing their expression levels. Transfection efficiencies were confirmed by WB detection of both β-arr isoforms (Figure 1b, left panel). Consistent with the pattern observed in β1/β2-MEF KO cells, β-arr2 depletion enhanced while β-arr1 silencing severely hindered receptor degradation (Figure 1b). In addition, in gain-of-function experiments the two β-arr isoforms demonstrate an equivalent opposing pattern: β-arr2 overexpression reduces, whereas β-arr1 overexpression enhances the rate of ligand-dependent degradation relative to mock-transfected cells (Figure 1b). Densitometric quantification of multiple experiments confirmed the diverging roles for the two isoforms in both silencing and overexpression conditions (Figure 1b, graphs). The same experiment performed in the human embryonic kidney cell line HEK293T extended the opposing roles of the two β-arr isoforms to cells of human background (Figure 1c). It should be noted that before stimulation (serum-starved cells), the levels of IGF-1R are decreased in conditions with β-arr2 overexpression, both in MEF and HEK293T cells (Figures 1b and c).
Taken together, these results suggest that in contrast to the documented role of β-arr1 in facilitating IGF-1R degradation, β-arr2 limits the rate of ligand-induced IGF-1R degradation.
Role of β-arrestin2 in IGF-1R-mediated signalling
The other distinctive role of β-arr1 in regulating IGF-1R function is to sustain its signalling and act as a transducer molecule by connecting the receptor with downstream cytoplasmic signalling complexes.27, 32, 39, 40 For the IGF-1R as well as for the larger class of GPCRs, the stability of the receptor–arrestin interaction controls the fate of the complex throughout the endocytic pathway, with a clear relationship between the short time effects (0–60 min) of ligand-induced signalling and longer effects (12–24 h) on degradation. A strong and durable interaction supports sustained ERK activity and eventual degradation, whereas a weak interaction is mirrored by transient ERK activation, favouring receptor recycling.13, 32, 33, 37 Thus, we next investigated the roles of the different β-arr isoforms in regulating the temporal characteristics of IGF-1 signalling. To separate the effects of the two isoforms, we first examined the dynamics of the two key IGF-1R downstream signalling pathways (Ras/Raf/MEK/ERK and PI3K/Akt) in MEF cells lacking either β-arr1 or β-arr2. Serum-starved cells were stimulated with IGF-1 (50 ng/ml) for up to 60 min and phosphorylated levels of the receptor, Akt and ERK1/2 were measured by WB as indicators of IGF-1R signalling activation. Although the phospho-IGF-1R and phospho-Akt levels were essentially similar in the cells expressing both β-arrs (WT) or only one isoform (β2KO, β1KO), the most dramatic, and statistically significant differences were observed in the ERK activation at the later (30–60 min) time points (Figure 2a). In all cell lines, ERK1/2 activation reaches maximal levels within 5 min of IGF-1 treatment. In WT cells, phospho-ERK rapidly decreases at 30 min, returning to basal levels ~60 min after stimulation. This decrease is much slower in cells expressing only β-arr1 (β2KO) with phospho-ERK levels at 30 and 60 min after stimulation approximately threefold higher compared to basal levels (Figure 2a, graph). In contrast, expression of β-arr2 alone (β1KO) leads to a very dynamic but transient ERK1/2 activation, which decreases rapidly after 10 min of IGF-1 treatment and reaches basal levels 30 min after stimulation (Figure 2a).
Role of β-arrestin2 in IGF-1R-mediated signalling. (a–c) MEFs knockout for either β-arr isoform (a) or MEF WT (b) and HEK293T (HEK) (c) transfected with isoform-specific β-arr2-encoding plasmid (+β2), siRNA (-β2) or respective controls (M), were serum starved and stimulated with IGF-1 (50 ng/ml) for 0–60 min. Lysates were analysed by WB for levels of phosphorylated (p) -IGF-1R, -AKT, -ERK, alongside total ERK and AKT, and GAPDH as a loading control (left panels). Late (30 and 60 min) ERK phosphorylation levels were quantified by densitometry, normalized to GAPDH and displayed as fold change from unstimulated level (graphs, right panel). Values indicate mean±s.e.m. from three independent experiments. Statistical analysis (two-tailed t-test): changes of IGF-1-dependent late pERK induced by β-arr modulation (KO, +, −) compared with their respective WT or M (empty vector/non-target siRNA) control, *P<0.05, **P<0.01, ***P<0.001.
To confirm the inhibitory role of β-arr2 on sustained IGF-1-induced ERK signaling, we used a transient transfection system to overexpress and silence the β-arr2 isoform in MEF WT cells. Verified transfection efficiency levels were similar to the ones displayed in Figure 1b.
Consistent with the results obtained with β2KO cells, β-arr2 silencing resulted in prolonged ERK activation, with their levels approximately threefold higher than basal levels 30 and 60 min after stimulation, while in the mock-transfected cells the activated ERK levels returned to the unstimulated levels about 30 min after IGF-1 treatment.
Overexpression of β-arr2 on the other hand reverses this phenomenon, and the late phospho-ERK profile is suppressed in comparison to mock transfected, confirming an inhibitory role for β-arr2 on IGF-1-induced ERK phosphorylation (Figure 2b). The same β-arr2 loss/gain-of-function experiment was performed with human HEK293T, revealing a similar pattern, with β-arr2 suppressing continued ERK activation, opposing β-arr1 sustained ERK activation (Figure 2c). Trends and statistical significance were confirmed by densitometry quantification of multiple independent experiments (Figure 2, graphs) and once more verify that the regulatory roles of β-arr2 in IGF-1R occur not only in mouse, but also in human background cells. Of note, the pIGF-1R level showed a slight tendency for increase after β-arr2 overexpression, yet this difference was not statistically significant across multiple independent experiments.
Altogether, these results suggest that levels of β-arr2-mediated ERK1/2 activation downstream of IGF-1R follows a very different time course from that mediated by β-arr1. Whereas β-arr1 sustained IGF-1-induced ERK activation,27 β-arr2 functionally antagonizes β-arr1 to limit MAPK action at later time points.
Dependency on Mdm2 of the β-arr2-mediated IGF-1R degradation and signalling
We have previously shown that β-arr1 is a key protein controlling receptor expression and MAPK activation through Mdm2-dependent IGF-1R ubiquitination.27, 28 The C terminal of the IGF-1R is essential for β-arr1 binding, which in turn connects the Mdm2 ubiquitin ligase to the activated receptor.24, 28, 32 Thus, we sought to evaluate whether there is a causative relationship between the IGF-1R/Mdm2 interaction and the observed effects of β-arr2. MEF cells knockout for IGF-1R and stably expressing a C-terminal truncated IGF-1R (Δ1245), were transfected with specific β-arr2 siRNA or overexpressing plasmid, and transfection efficiency verified by WB (Figure 3a). Transfected, serum-starved cells were either stimulated with IGF-1 for up to 24 h to monitor the IGF-1R degradation rate or for up to 60 min to display the signalling activation. Unlike WT IGF-1R in the same MEF background (Figure 1), Δ1245 IGF-1R was insensitive to either reducing or overexpressing β-arr2 as demonstrated by an unchanged degradation rate (Figure 3a, upper panel). Likewise, the Δ1245 IGF-1R signalling activity as measured by phosphorylated levels of IGF-1R, ERK and Akt in response to IGF-1 was essentially unchanged by β-arr2 modulation (Figure 3b). Additional control of the total levels of ERK and Akt demonstrated that they were unchanged by transfection or stimulation (Supplementary Figure 1A). These results suggest that β-arr2, similar to β-arr1, mediates its effects through the Mdm2-interacting domain of the IGF-1R.
Dependency on Mdm2 of the β-arr2-mediated IGF-1R degradation and signalling. (a, b) MEF expressing truncated IGF-1R, defective in binding Mdm2 (MEF 1245), transfected with isoform-specific β-arr2-encoding plasmid (+β2), siRNA (−β2) or respective control (M) were lysed and level of β-arr2 verified by WB, using GAPDH as a loading control (a, left panel). Transfected cells were serum starved and stimulated with IGF-1 (50 ng/ml) for 0, 12 or 24 h. Lysates were analysed by WB for IGF-1R, quantified by densitometry, normalized to GAPDH and displayed as percentage of mock unstimulated controls (a). Parallel samples were stimulated for 0–60 min and analysed by WB for phosphorylated (p) -IGF-1R, -AKT, -ERK and GAPDH. Late (30 and 60 min) ERK phosphorylation levels were quantified by densitometry, normalized to GAPDH and displayed as fold change from unstimulated level (graphs, right panel). Values indicate mean±s.e.m. from three independent experiments. (c, d) U2OS and SAOS2, transiently transfected with isoform-specific β-arr2-encoding plasmid (+β2), siRNA (−β2) or respective control (M) were lysed and levels of β-arr2 level verified by WB, using GAPDH as a loading control (c, left panel). Transfected cells were serum starved and stimulated with IGF-1 (50 ng/ml) for 0, 12 or 24 h. Lysates were analysed by WB for IGF-1R, quantified by densitometry, normalized to GAPDH and displayed as percentage of mock unstimulated controls (c, right panel, graphs). Parallel samples were stimulated for 0–60 min and analysed by WB for phosphorylated (p) -IGF-1R, -AKT, -ERK and GAPDH. Late (30 and 60 min) ERK phosphorylation levels were quantified by densitometry, normalized to GAPDH and displayed as fold change from unstimulated level (graphs, right panel). Values indicate mean±s.e.m. from three independent experiments (d). Statistical analysis (two-tailed t-test): IGF-1-induced degradation rate following β-arr modulation (+, −) compared with their respective M (empty vector/non-target siRNA) controls (a, c). Changes of IGF-1-dependent late pERK induced by β-arr modulation (+, −) compared with their respective M (empty vector/non-target siRNA) control (b, d). *P<0.05, ***P<0.001.
To explore in greater detail the dependency of β-arr2 on the Mdm2 system, two human osteosarcoma cell lines, U2OS and SAOS-2, were used. The rationale behind this choice is that the expression level of Mdm2 differs considerably in these two cell lines: U2OS expresses high levels of Mdm2, whereas SAOS-2 is p53 negative and therefore exhibits low Mdm2 expression levels.41, 42, 43 Both cell lines were transfected with β-arr2 plasmid or β-arr2 siRNA and transfection efficiency confirmed by WB (Figure 3c, left panel). In U2OS, β-arr2 inhibition enhanced IGF-1R degradation rate while β-arr2 overexpression stabilizes receptor levels, consistent with the results obtained in the other tested cells expressing both Mdm2 and full-length IGF-1R (Figure 3c). SAOS-2 on the other hand, expressing low Mdm2, showed no significant difference in IGF-1-induced degradation rates following β-arr2 modulations (Figure 3c). Once more, as with MEF and HEK293T cell lines, β-arr2 overexpression decreased IGF-1R basal levels both in U2OS and SAOS-2 cells.
We also explored the possible dependency of β-arr2-mediated IGF-1R signalling on Mdm2 by stimulating the cells with IGF-1 for up to 60 min (Figure 3d). In U2OS cells, ERK phosphorylation in response to IGF-1 was extended in conditions with decreased β-arr2 and shortened by β-arr2 overexpression (Figure 3d, graph), whereas the same signalling pathway was essentially unchanged in SAOS-2 cells. In all transfection conditions, levels of total Akt and ERK were unchanged throughout stimulation (Supplementary Figure 1B).
Taken together, these results indicate that the β-arr2 effects on IGF-1R degradation and signalling are dependent on the presence of Mdm2 and its interaction with the IGF-1R C terminus, pointing towards Mdm2-mediated ubiquitination of the IGF-1R as a potential mediating mechanism.
β-arrestin2/IGF-1R interaction and receptor ubiquitination as the underlying mechanism controlling IGF-1R expression and signalling
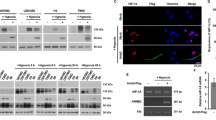
Previous in vitro experiments indicated that β-arr1 is more efficient than β-arr2 in inducing IGF-1R ubiquitination,28 and established β-arr1 recruitment to the IGF-1R as an essential step controlling trafficking and signalling through ligand-dependent ubiquitination of the receptor.24, 28, 32 Thus, we next questioned whether a similar sequence of events is true for β-arr2 by comparing how the two isoforms interact with the IGF-1R, affect receptor ubiquitination, and the ligand dependency of these behaviours. As an experimental system, we used the U2OS cell line, as they overexpress functional Mdm2.41 Cells transfected with Flag-tagged versions of either of the β-arr isoforms, serum starved and stimulated or not with IGF-1, were lysed and the Flag-tagged proteins immunoprecipitated. The obtained β-arr-immuno-precipitates were analysed by WB for the presence of the β-subunit of IGF-1R (Figure 4a). The Flag-tagged protein and IGF-1R levels in the lysates prior to precipitation, as well as the amounts of Flag protein captured by the anti-Flag beads are shown (Figure 4a).
β-arrestin2/IGF-1R interaction and receptor ubiquitination as the underlying mechanism controlling IGF-1R expression and signalling. (a) U2OS cells transfected with isoform-specific Flag-tagged-β-arr-encoding plasmids (+β1/+β2) were serum starved and stimulated with IGF-1 (50 ng/ml) for 0–60 min. β-arrs were immunoprecipitated (IP) via their Flag-tag and association with IGF-1R detected by WB, with Flag detection used as loading controls. Expression of IGF-1R, Flag and GAPDH in the total cell lysates (TCL) before IP were anaysed by WB. (b) U2OS cells co-transfected with IGF-1R and Flag-tagged-β-arr-encoding plasmids (+β1/+β2), were serum starved, stimulated with IGF-1 (50 ng/ml) for 0–60 min, and immunoprecipitated via their Flag tag. WB detection analysed level of IGF-1R in the immuno-precipitates. Expression of IGF-1R, Flag and GAPDH in the TCL before IP were analysed by WB. (c) U2OS cells co-transfected with IGF-1R and Flag-tagged-β-arr-encoding plasmids (+β1/+β2), were serum starved, stimulated with IGF-1 (50 ng/ml) for 0–60 min, and immunoprecipitated via IGF-1R. WB detection analysed level of ubiquitin (Ub) in the immuno-precipitates. Expression of IGF-1R, Flag and GAPDH in the TCL before IP were analysed by WB.
Both Flag-tagged β-arr isoform immuno-precipitates contained clearly detectable levels of IGF-1R, however, the β-arr1/IGF-1R association lasted longer while β-arr2 disengages earlier from the ligand-occupied IGF-1R (Figure 4a). Densitometry quantification of multiple experiments demonstrates that the IGF-1R/β-arr2 dissociation reaches statistical significance 5 min after stimulation, compared with 60 min for β-arr1 (Supplementary Figure 2). Attempts to verify the resultant ubiquitination of endogenous IGF-1R was unsuccessful due to high inter-experimental variability of the IGF-1R levels after β-arr overexpression, therefore, we co-transfected the cells with IGF-1R overexpressing plasmids (Figure 4b). In conditions where both IGF-1R and either β-arr isoform were overexpressed, β-arr2 demonstrated a higher affinity for the unstimulated receptor. Addition of IGF-1 clearly increases β-arr1/IGF-1R association but rapidly disengages β-arr2, evident even by 2 min after stimulation (Figure 4b). These patterns were verified by quantification of multiple independent experiments (Supplementary Figure 2). The lower panels confirm Flag-tagged protein and IGF-1R levels in the lysates prior to precipitation and the amounts of Flag protein captured (Figure 4b). Parallel samples were used for IGF-1R immuno-precipitation to verify the resultant ubiquitination status of the receptor in each condition, using IGF-1R-only transfected cells as control (Figure 4c). In line with previous studies,24, 28 increasing β-arr1 recruitment to the IGF-1R enhanced both the basal and ligand-stimulated receptor ubiquitination. On the other hand and in line with the observed IGF-1R association pattern, an increased level of β-arr2 also increased unstimulated IGF-1R ubiquitination, which remained unchanged upon commencement of ligand stimulation, resulting in an overall dampening of levels of receptor ubiquitination when compared with the mock or β-arr1-transfected cells (Figure 4c).
Together, these data suggest that in comparison to β-arr1, the β-arr2 isoform has a higher affinity for the unstimulated receptor, where it maintains a basal ubiquitination level, dissociates faster following ligand stimulation delaying ligand-dependent IGF-1R degradation.
Effects of β-arrestin2 on IGF-1-dependent proliferation
So far, our results demonstrate that β-arr2 functionally antagonizes β-arr1 by inhibiting IGF-1R ubiquitination and degradation, as well as limiting MAPK/ERK signalling activation. The MAPK/ERK pathway is critical for IGF-1-induced cell cycle progression and proliferation,27, 44, 45 therefore, we investigated the impact of β-arr2 in this process. The β-arr1/β-arr2 status quo in U2OS and SAOS-2 was unbalanced by transfecting either β-arr1/β-arr2 plasmid or β-arr1/β-arr2 siRNA, and transfection efficiency confirmed by WB (Figure 5a). SAOS-2 cells express low endogenous levels of β-arr1, and hence suppression was at the boundary of WB detection, therefore, qPCR was performed and confirmed at least 80% knockdown. Serum-starved cells, stimulated with and without IGF-1 for 24 h, were analysed by FACS for cell cycle phase distribution and equivalent samples were evaluated for total cell number (Figures 5b and c). Both U2OS and SAOS-2 cells respond to IGF-1 stimulation by moving from a G0/G1 phase into an S/G2 phase, indicative of cell cycle progression (Figure 5b), and which translated into a corresponding increase in cell number (Figure 5c). β-arr1 overexpression or siRNA knockdown of β-arr2 in either cell lines slightly increases IGF-1-mediated cell cycle progression (Figure 5b) and total cell number (Figure 5c); however, these trends reach statistical significance only in U2OS cells with the -β-arr2 condition. On the other hand, β-arr2 overexpression or β-arr1 inhibition yields the most critical, statistically significant changes, making the cells unresponsive to IGF-1 and drastically reducing the total cell number (Figures 5b and c). Intriguingly, the pattern of cell cycle arrest was different between the two cell lines: while U2OS cells display both G1 and G2/M arrest, and are ultimately insensitive to IGF-1 stimulation, SAOS-2 cells respond to IGF-1 by successfully entering the cell cycle but appear unable to complete mitosis, illustrated by a G2 phase predominance enlarged by IGF-1. Evaluation of total cell number (Figure 5c) mirrored the effects revealed by FACS analysis: β-arr2 overexpression or β-arr1 inhibition drastically decreases the total cell number, with the SAOS-2 cells being preferentially affected.
Effects of β-arrestin2 on IGF-1-dependent proliferation. (a) U2OS and SAOS2 were transfected with isoform-specific β-arr-encoding plasmids (+β1/+β2), siRNAs (−β1/−β2) or respective controls (M), and transfection efficiency was assayed by WB, with GAPDH as a loading control. (b, c) Transfected cells stimulated or not with IGF-1 (50 ng/ml) for 24 h were analysed for cell cycle distribution (G1, S and G2/M) (b) or cell viability (c). Results from three independent experiments are displayed as mean±s.e.m., percentage of total cell population (b) or as a mean±s.e.m. relative to unstimulated M controls (c). Statistical analysis (two-tailed t-test): IGF-1-induced cell cycle progression (b) or proliferation (c) following β-arr modulation (+, −) compared with their respective M (empty vector/non-target siRNA) controls. **P<0.01, ***P<0.001.
These results suggest that sustained β-arr1-MAPK signalling, antagonized by β-arr2, is required for entering and completing G1/S transition in U2OS cells as well as for completing G2/M transition in both cell lines.
Resultant p53 cancer relevant effects of unbalanced β-arrestin1/β-arrestin2
How does unbalancing β-arr1/β-arr2 equilibrium towards the latter decrease cell number, cause cells to preserve or fail in their response to IGF-1 and halt proliferation at different points of the cell cycle? We considered two possible scenarios: first, that β-arr2 is more effective at downregulating the IGF-1R in serum-free conditions in U2OS as compared with SAOS-2 cells. In this case, downregulation of IGF-1R, prior to ligand stimulation would make U2OS cells insensitive to IGF-1. The second possibility is that the disparate G1/S cell cycle arrest of U2OS and SAOS-2 after β-arr2 overexpression/β-arr1 inhibition may be due to their difference in p53 status, as SAOS-2 cells are null for p53, whereas U2OS have WT p53.
Along such lines, we examined the first scenario by studying the effects of β-arr1/β-arr2 modulation on IGF-1R expression while maintaining the cells in serum-free media for the entire duration of the experiment. β-arr1 inhibition or β-arr2 overexpression decreased receptor levels in both cell lines (Figure 6a), yet the SAOS-2 cells were much more sensitive in this respect, as well as in regards to overall cell survival: they could not survive longer than 72 h after transfection. In the absence of IGF-1 stimulation, β-arr1 overexpression also decreased the receptor levels in both cell lines, yet the onset of these effects were delayed ~24 h compared to conditions with increased β-arr2 and were less detrimental to overall cell survival in both cell lines. We next evaluated the second scenario, by specifically testing the U2OS cells for p53 level in response to IGF-1 stimulation. Ligand-activated IGF-1R resulted in decreased p53 levels in conditions with functionally selected β-arr1 signalling (that is, β-arr1 overexpression or β-arr2 inhibition) (Figure 6c). Once more, β-arr2 prevalence plays an opposite role and reverses this pattern by increasing p53 levels despite IGF-1 stimulation.
Resultant p53 cancer relevant effects of unbalanced β-arrestin1/β-arrestin2. (a, b) U2OS and SAOS2 transfected with isoform-specific β-arr-encoding plasmids (+β1/+β2), siRNAs (−β1/−β2) or respective controls (M), were cultured in serum-free media (SFM) conditions, and samples collected at indicated times. Lysates were analysed by WB for IGF-1R, with GAPDH as a loading control. (c) U2OS cells, transfected as indicated were serum starved and stimulated with IGF-1 (50 ng/ml) for 0, 12 or 24 h. Lysates were analysed by WB for p53, with GAPDH as a loading control. WBs were quantified, normalized to GAPDH and displayed as percentage of mock (M) unstimulated (0 h) control. Results from three independent experiments are shown as mean±s.e.m. Statistical analysis (two-tailed t-test): IGF-1-induced changes in p53 expression following β-arr modulation (+, −) compared with their respective M (empty vector/non-target siRNA) controls, *P<0.05, **P<0.01, ***P<0.001.
These results suggest that both scenarios overlap to explain the behaviour of cancer cells with predominant β-arr2 control: IGF-1 insensitivity, secondary to receptor downregulation in the serum starvation phase could contribute to the decrease in the overall cell number; in cells with functional p53, increase in its expression levels, parallel the G1/S cell cycle arrest.
Discussion
The last decades have witnessed the recognition of the β-arr system as a major hub controlling nearly the entire GPCR signalling network. β-arrs are now known to orchestrate many GPCR functions, including receptor desensitization, trafficking2, 3, 46 and signal transduction12, 13, 47, 48, 49, 50 eventually directing the GPCR’s biological effects.51, 52 As both β-arrs coordinate the signalling cascades downstream of activated receptors, the antagonism versus complementarity of the two isoforms represents an essential layer of combined safe control and specialization. The high degree of sequence similarity provides an explanation of how β-arr isoforms complement each other,53, 54 while crystallographic, biophysical and proteomic studies begin to reveal the mechanisms underlying their functional divergence. These include the conformational differences, interactions with other proteins and subcellular localization (for extensive reviews, see refs 55, 56, 57), with the affinity of the GPCR/β-arr association emerging as a critical controller of receptor trafficking and signalling. On the basis of this criteria, most GPCRs can be classified into two groups.58 Class A receptors (for example, β2 adrenergic receptor10, 48 and the dopamine D1A receptor58) bind β-arr2 with greater affinity but transiently, recycle rapidly, and mediate short β-arr-dependent MAPK signalling. Class B receptors, on the other hand, (for example, the angiotensin II type 1A receptor59 and the vasopressin V2 receptor50, 60) bind both β-arr isoforms with equal affinity, recycle slowly, remain bound to their β-arr through trafficking to endosomes, and hence mediate a longer β-arr-dependent MAPK signal.39, 58 Furthermore, at any one receptor, both β-arr isoforms can be required for ERK signalling (termed codependence), or one isoform activates ERK signalling while the other inhibits it (termed reciprocal regulation).59
Although for GPCRs the functional partnership between β-arr isoforms is acknowledged to be relevant for various physiological and pathological processes and so for therapeutic targeting,55, 61, 62 little is known about this process in the case of receptor tyrosine kinases. Hence, the first main finding of the present study reveals the functional antagonism between β-arr isoforms in relation to the IGF-1R, a prototypical receptor tyrosine kinase. This opposing behaviour was demonstrated for the two main roles known for β-arr1 at the IGF-1R. First, our results revealed that β-arr2 acts to protect the receptor from ligand-induced degradation, opposing the IGF-1R depletion enhanced by β-arr1. In conditions with low or absent IGF-1, overexpression of either β-arr1 or β-arr2 downregulates the receptor, yet for the latter, this effect is faster and more prominent. Second, our data exposes a reciprocal regulation pattern with clear antagonism between isoforms in relation to signalling: β-arr2 balances against β-arr1 sustained MAPK/ERK activity induced by IGF-1. Of note, this feature was not present in SAOS-2 cells, which have low Mdm2 levels. The functional analysis of IGF-1R/β-arr interactions demonstrated that both isoforms interact with the IGF-1R via its C-terminal tail, promoting Mdm2-mediated ubiquitination. Conditions with absent or low IGF-1 favour a GPCR class A-like, transient β-arr2-recruitment pattern, while IGF-1R conformational changes induced by ligand stimulation mirrored a GPCR class B-like pattern with an increased, stabilized receptor/β-arr1 interaction that in turn boosts Mdm2-dependent IGF-1R ubiquitination. Furthermore, our data exposed an intriguing β-arr antagonism downstream of IGF-1R on p53 activation: while β-arr1 sustains low p53 levels, β-arr2 acts to limit these effects. This is remarkable as it has been recently demonstrated that in response to β2-adrenoreceptors activation, β-arr1 moves to the nucleus where it functions as an adaptor protein to promote the binding and degradation of p53 by the E3-ubiquitin ligase Mdm2, allowing accumulation of DNA damage.63, 64
One fundamental question remains: what mechanisms control the functional divergence of β-arr isoforms downstream of IGF-1R? One potential mechanistic insight is provided by the clear correspondence between the effects on ERK signalling, ubiquitination and degradation, and implies different affinities of the receptor for each β-arr isoform, controlled by ligand-induced conformational changes. In this model (Figure 7), we propose that β-arr2 has greater affinity than β-arr1 for the ligand-unoccupied receptor, as demonstrated by the co-immunoprecipitation studies. Although this interaction is short lived, it can trigger receptor ubiquitination and degradation, without effects on MAPK activation. Conversely, the ligand-occupied receptor has a greater affinity for β-arr1, the association lasts longer, suppresses p53 levels and activates MAPK signalling at the cost of increased receptor degradation. Yet, all these effects can be reversed by modifying local β-arr concentrations, suggesting a possible direct competition between the two isoforms, determined by their relative abundance. Such a mechanism could be particularly relevant for the observed functional antagonism on p53 levels as it has been reported that βarr2 sequesters βarr1 in the cytosol through hetero-dimerization, thus preventing its nuclear translocation and p53 degradation.56, 64, 65, 66
Functional divergence of β-arr isoforms at the IGF-1R. (a) The agonist-unstimulated IGF-1R has a greater affinity for β-arr2 than β-arr1, initiating a low level of Mdm2-dependent IGF-1R ubiquitination, which in β-arr2 predominance conditions leads to ligand-independent degradation. β-arr2 in the cytoplasm sequesters β-arr1 by forming biologically inactive heterodimers. (b) Ligand-induced receptor conformational changes cause a switch in affinity towards the β-arr1 isoform, mediating stable β-arr1/IGF-1R association, enhanced Mdm2-dependent ubquitination and receptor internalization. In conditions with β-arr2 predominance, β-arr2 dissociates faster from the ligand-occupied receptor, which delays ligand-dependent receptor degradation through recycling pathways. (c) A strong and prolonged β-arr1/IGF-1R interaction sustains ERK activity and eventual degradation, whereas the weaker interaction of β-arr2/IGF-1R is mirrored by transient ERK activation (d). β-arr1 signalling predominance maintains low p53 levels, allowing cell cycle progression. β-arr2 opposes this process, possibly by sequestering both β-arr1 and Mdm2 in the cytoplasm, rendering cell cycle arrest.
As the second key finding, the present study identifies the antagonism between the two β-arr isoforms in controlling IGF-1R as a potential target for cancer therapy. As proof of concept, we demonstrated contrasting abilities of IGF-1R to interact with each β-arr isoform, depending on the presence of the ligand or local concentrations of β-arr isoforms. Biasing this system towards β-arr2 leads to decreased viability of cancer cells, unable to complete the G1/S transition or complete mitosis.
Cell proliferation can be induced by either prolonged exposure to mitogens or a two-pulse sequential stimulation. A first signalling wave, which includes ERK, primes cells into early G1 while activating a p53-restraining response.67 A second pulse of mitogens generates sustained MAPK signalling and removes the p53 constraint, allowing the cells to complete the cell cycle. In U2OS, in the relative absence of β-arr1 signalling downstream of IGF-1R (due to β-arr1 inhibition or β-arr2 overexpression), p53 accumulates and halts the cells in G1. As the cells already in S/G2 are unable to exit the cell cycle, this indicates β-arr1 signalling as essential for cell cycle completion. On the other hand, in the same conditions, SAOS-2 cells bypass the G1 checkpoint when stimulated by IGF-1, because no p53 is present to halt them, yet arrest in G2 and are unable to complete the cycle. The corollary of this data is that β-arr1-mediated signalling downstream of IGF-1R is required to remove p53 constraints and allow entry into S-phase as well as for completion of G2/M phase. Together with the effects on IGF-1R expression and signalling, this model explains the functional outcome of targeted β-arr1/2 imbalance towards the latter in cancer cells: lack of responsiveness to IGF-1 and cell cycle arrest, leading to decreased viability.
With the present study, we highlight the opposing roles of β-arr isoforms to modulate IGF-1R expression and function. The β-arr1/2 balance plays a key role in cell cycle checkpoint progression, even in instances where p53 is absent. The manipulation of this system carries strong therapeutic potential by acting to both inhibit a pro-tumourigenic proliferative signal, and also to enhance anti-tumourigenic apoptotic pathway.
Materials and methods
Cell culture
HEK293T, SAOS-2 and U2OS cell lines were obtained from ATCC (via LGC Standards, Middlesex, UK) and grown in DMEM (HEK293T) or IMDM (U2OS and SAOS-2) medium supplemented with 10% (vol/vol) FBS and 1% penicillin/streptomycin (P/S). MEF Δ1245 (IGF-1R knockout MEF cells, stably transfected with IGF-1R containing a C terminus truncation at position 1245) were kindly provided to us by Dr R Baserga (Thomas Jefferson University, Philadelphia, PA, USA)68 and were cultured in DMEM supplemented with 10% FBS and 1% P/S in the presence of G-418 (Promega Biotech AB, Nacka, Sweden). MEFs from wild type or from β-arrestin1 or β-arrestin2 knockout mice were kindly provided to us by Dr RJ Lefkowitz (Howard Hughes Medical Institute, Durham, NC, USA),5, 6 and were cultured in DMEM medium supplemented with 10% FBS and 1% P/S. All human cell lines were validated by short tandem repeat profiling of extracted genomic DNA (Uppsala Genome Centre, Uppsala, Sweden). Mouse cell lines were authenticated by examination of growth characteristics and expression of truncated IGF-1R or knockout of β-arrestin isoform by WB. All cell lines were tested for mycoplasma contamination regularly.
Transfection
Small interfering RNAs (siRNAs) targeting β-arrestin1 (Human ARRB1 (408) #LU01197100, Mouse ARRB1 (109689) #LU0409700) and β-arrestin2 (Human ARRB2 (409) #LU0072900, Mouse ARRB2 (216869) #LU04102201) were purchased from GE Dharmacon via Thermo Fisher Scientific (Göteborg, Sweden). For both human and mouse cells, a non-target siRNA (D00181001) was used as a control. The cells were transfected at 40–50% confluency in six-well plates, using Lipofectamine RNAiMAX (Invitrogen via Thermo Fisher Scientific) according to the manufacturer’s instructions.
The Flag-tagged β-arrestin1 and β-arrestin2 plasmids in pcDNA3 were kindly provided to us by Dr RJ Lefkowitz (Duke University Medical Center/Howard Hughes Medical Institute, Durham, NC, USA).53, 69 Cells were plasmid transfected using Turbofect (Thermo Fisher Scientific) according to the manufacturer’s instructions. Empty vector (pcDNA3) transfections were used as controls for plasmid overexpression. All transfection experiments were verified for efficiency by WB at 24 h after transfection.29, 32
qPCR
Transfection efficiency for β-arrestin1 silencing in SAOS-2 and U2OS (Figure 5) was confirmed by qRT–PCR, as described in detail elsewhere.70 Specific human probes (Hs00244527_m1) for β-arrestin1 were used for real-time qPCRs, performed with the StepOne Plus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA).
IGF-1 stimulation
Prior to IGF-1 stimulation experiments (long-term degradation or short-term signalling), attached cells were washed with PBS and changed to serum-free medium and incubated at 37 °C for 8–12 h. Stimulation was preformed using recombinant human IGF-1 (Sigma-Aldrich Ltd., St Louis, MO, USA) at 50 ng/ml.
Immunoprecipitation
After indicated treatments, cells cultured in six-well plates were lysed with 600 μl Pierce IP Lysis buffer (Thermo Scientific) containing protease inhibitor cocktails (Thermo Fisher Scientific). When detecting ubiquitination of IGF-1R, 10 mM N-ethylmaleimide was additionally added to the lysis buffer. Protein concentration was determined by bicinchoninic acid assay (Thermo Fisher Scientific). Equivalent amounts of lysates were incubated with 10 μl of anti-Flag agarose beads or 1 μg of IGF-1R antibodies overnight at 4 °C and with 10 μl of Protein G agarose beads (GE Healthcare, Amersham, UK) for 2 h at 4 °C. The immunoprecipitates were collected by centrifugation, the pellet was washed four times with lysis buffer and then dissolved in a sample buffer for SDS/PAGE and was further analysed by WB.
SDS–PAGE and WB
Protein samples were dissolved in lithium dodecyl sulfate sample buffer and analysed by SDS–PAGE with 4–12% Bis-Tris gels (Invitrogen, via Thermo Fisher Scientific). Upon separation, the proteins were transferred to nitrocellulose membranes, blocked for 1 h in bovine serum albumin and 0.1% Tween 20 in tris-buffered saline (TBS), followed by overnight incubation with primary antibody at 4 °C. Antibodies against phosphorylated(p)Akt (#4060), pERK1/2 (#9101), ERK1/2 (#9102), pIGF-1R (#3021), IGF-1R (#3027) and β-arrestin1/2 (#4674S) were from Cell Signaling Technology (via BioNordika, Stockholm, Sweden) and were all used at 1:2000 dilution in bovine serum albumin. Ubiquitin (P4D1) (sc-8017), p53 (sc-126) and GAPDH antibodies (sc-25778) were from Santa Cruz Biotechnology (Heidelberg, Germany) and were used at 1:2000 in milk. Following 3 × 10 min washing (TBS-T), membranes were incubated with secondary antibody, either fluorescence-conjugated IRDye (LI-COR Biosciences, Cambridge, UK) and detection with LI-COR Odyssey (LI-COR Biosciences, Cambridge, UK) or horseradish peroxidase-conjugated and detection with ECL substrate (Pierce via Thermo Fisher Scientific) and exposure to X-ray film.37
Quantification of WBs
Western transfer analysis bands were quantified using the ImageJ programme (http://rsbweb.nih.gov/ij/) or Image Studio (LI-COR Biosciences, Cambridge, UK), subtracting the background level.
Cell viability/proliferation
Cells were incubated with PrestoBlue (Life Technologies, Carlsbad, CA, USA) reagent for 30 min and the fluorescence from excitation at 560 nm and emission at 590 nm was measured using a TECAN Infinite 1000 plate reader. A standard curve was used to interpolate fluorescence to cell number.
Cell cycle distribution analysis
Following treatment, cells were collected and centrifuged at 1500 rpm for 4 min to pellet, resuspended dropwise in 0.5 ml of 95% ethanol and incubated for >1 h. Cells were rehydrated in water and treated with protease subtilisin and nuclei were then stained with DAPI for 30 min before analysis by FACS.71
Statistical analysis
Where indicated, data from a minimum of three independent experimental replicates of two conditions were compared using a two-tailed, unpaired t-test assuming equal variance. As part of experimental design, before performing the experiment, a threshold value of P=0.05 was chosen for testing the null hypothesis. The variances of experimental groups that are being compared were not statistically different. Data expressed with error bars show mean±s.e.m. from three independent biological experiments, unless otherwise stated. Significance is given as *P<0.05, **P<0.01, ***P<0.001.
References
Lefkowitz RJ . Arrestins come of age: a personal historical perspective. Prog Mol Biol Transl Sci 2013; 118: 3–18.
Shenoy SK, Lefkowitz RJ . Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J 2003; 375 (Pt 3): 503–515.
Shenoy SK, Lefkowitz RJ . Beta-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 2011; 32: 521–533.
DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK . Beta-arrestins and cell signaling. Annu Rev Physiol 2007; 69: 483–510.
Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE et al. Beta-arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 1997; 81: 1021–1026.
Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT . Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 1999; 286: 2495–2498.
Zhang M, Liu X, Zhang Y, Zhao J . Loss of betaarrestin1 and betaarrestin2 contributes to pulmonary hypoplasia and neonatal lethality in mice. Dev Biol 2010; 339: 407–417.
Lee MH, El-Shewy HM, Luttrell DK, Luttrell LM . Role of beta-arrestin-mediated desensitization and signaling in the control of angiotensin AT1a receptor-stimulated transcription. J Biol Chem 2008; 283: 2088–2097.
Sanni SJ, Hansen JT, Bonde MM, Speerschneider T, Christensen GL, Munk S et al. Beta-arrestin 1 and 2 stabilize the angiotensin II type I receptor in distinct high-affinity conformations. Br J Pharmacol 2010; 161: 150–161.
Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ . Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001; 294: 1307–1313.
Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 2003; 100: 10782–10787.
Lefkowitz RJ, Shenoy SK . Transduction of receptor signals by beta-arrestins. Science 2005; 308: 512–517.
Shenoy SK, Lefkowitz RJ . Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE 2005; 2005: cm10.
Hupfeld CJ, Olefsky JM . Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. Annu Rev Physiol 2007; 69: 561–577.
Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol 1994; 14: 3604–3612.
Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R . Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci USA 1993; 90: 11217–11221.
Baserga R . The IGF-I receptor in cancer research. Exp Cell Res 1999; 253: 1–6.
Baserga R . The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res 1995; 55: 249–252.
Economou MA, Andersson S, Vasilcanu D, All-Ericsson C, Menu E, Girnita A et al. Oral picropodophyllin (PPP) is well tolerated in vivo and inhibits IGF-1R expression and growth of uveal melanoma. Invest Ophthalmol Vis Sci 2008; 49: 2337–2342.
Economou MA, Wu J, Vasilcanu D, Rosengren L, All-Ericsson C, van der Ploeg I et al. Inhibition of VEGF secretion and experimental choroidal neovascularization by picropodophyllin (PPP), an inhibitor of the insulin-like growth factor-1 receptor. Invest Ophthalmol Vis Sci 2008; 49: 2620–2626.
LeRoith D, Roberts CT Jr . The insulin-like growth factor system and cancer. Cancer Lett 2003; 195: 127–137.
Larsson O, Girnita A, Girnita L . Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer 2005; 92: 2097–2101.
Girnita L, Girnita A, Brodin B, Xie Y, Nilsson G, Dricu A et al. Increased expression of insulin-like growth factor I receptor in malignant cells expressing aberrant p53: functional impact. Cancer Res 2000; 60: 5278–5283.
Girnita L, Girnita A, Larsson O . Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA 2003; 100: 8247–8252.
Vasilcanu D, Weng WH, Girnita A, Lui WO, Vasilcanu R, Axelson M et al. The insulin-like growth factor-1 receptor inhibitor PPP produces only very limited resistance in tumor cells exposed to long-term selection. Oncogene 2006; 25: 3186–3195.
Vasilcanu R, Vasilcanu D, Sehat B, Yin S, Girnita A, Axelson M et al. Insulin-like growth factor type-I receptor-dependent phosphorylation of extracellular signal-regulated kinase 1/2 but not Akt (protein kinase B) can be induced by picropodophyllin. Mol Pharmacol 2008; 73: 930–939.
Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Vasilcanu D, Girnita A et al. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem 2007; 282: 11329–11338.
Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Girnita A, Lefkowitz RJ et al. {beta}-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem 2005; 280: 24412–24419.
Zheng H, Shen H, Oprea I, Worrall C, Stefanescu R, Girnita A et al. beta-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing's sarcoma. Proc Natl Acad Sci USA 2012; 109: 20620–20625.
Crudden C, Girnita A, Girnita L . Targeting the IGF-1R: the tale of the tortoise and the hare. Front Endocrinol (Lausanne) 2015; 6: 64.
Crudden C, Ilic M, Suleymanova N, Worrall C, Girnita A, Girnita L . The dichotomy of the Insulin-like growth factor 1 receptor: RTK and GPCR: friend or foe for cancer treatment? Growth Horm IGF Res 2015; 25: 2–12.
Zheng H, Worrall C, Shen H, Issad T, Seregard S, Girnita A et al. Selective recruitment of G protein-coupled receptor kinases (GRKs) controls signaling of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA 2012; 109: 7055–7060.
Girnita L, Takahashi SI, Crudden C, Fukushima T, Worrall C, Furuta H et al. Chapter seven - when phosphorylation encounters ubiquitination: a balanced perspective on IGF-1R signaling. Prog Mol Biol Transl Sci 2016; 141: 277–311.
Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Touhara K, Lefkowitz RJ . G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem 1995; 270: 16495–16498.
Hallak H, Seiler AEM, Green JS, Ross BN, Rubin R . Association of heterotrimeric G(i) with the insulin-like growth factor-I receptor - release of G(beta gamma) subunits upon receptor activation. J Biol Chem 2000; 275: 2255–2258.
Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM . Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem 2001; 276: 15688–15695.
Girnita A, Zheng H, Gronberg A, Girnita L, Stahle M . Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene 2012; 31: 352–365.
Girnita L, Worrall C, Takahashi S, Seregard S, Girnita A . Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci 2014; 71: 2403–2427.
Lefkowitz RJ, Rajagopal K, Whalen EJ . New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 2006; 24: 643–652.
Povsic TJ, Kohout TA, Lefkowitz RJ . Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 2003; 278: 51334–51339.
Florenes VA, Maelandsmo GM, Forus A, Andreassen A, Myklebost O, Fodstad O . MDM2 gene amplification and transcript levels in human sarcomas: relationship to TP53 gene status. J Natl Cancer Inst 1994; 86: 1297–1302.
Sehat B, Andersson S, Girnita L, Larsson O . Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis. Cancer Res 2008; 68: 5669–5677.
Sehat B, Andersson S, Vasilcanu R, Girnita L, Larsson O . Role of ubiquitination in IGF-1 receptor signaling and degradation. PLoS One 2007; 2: e340.
Weber JD, Raben DM, Phillips PJ, Baldassare JJ . Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J 1997; 326 (Pt 1): 61–68.
Hoshino R, Tanimura S, Watanabe K, Kataoka T, Kohno M . Blockade of the extracellular signal-regulated kinase pathway induces marked G1 cell cycle arrest and apoptosis in tumor cells in which the pathway is constitutively activated: up-regulation of p27(Kip1). J Biol Chem 2001; 276: 2686–2692.
Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA et al. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem 1997; 272: 31051–31057.
Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A 2005; 102: 1442–1447.
Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999; 283: 655–661.
McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 2000; 290: 1574–1577.
Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ . Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA 2005; 102: 1448–1453.
Reiter E, Ahn S, Shukla AK, Lefkowitz RJ . Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 2012; 52: 179–197.
Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ . Recent developments in biased agonism. Curr Opin Cell Biol 2014; 27: 18–24.
Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 2014; 512: 218–222.
Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC et al. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 2013; 497: 137–141.
Srivastava A, Gupta B, Gupta C, Shukla AK . Emerging functional divergence of beta-arrestin isoforms in GPCR function. Trends Endocrinol Metab 2015; 26: 628–642.
Gurevich VV, Gurevich EV . Structural determinants of arrestin functions. Prog Mol Biol Transl Sci 2013; 118: 57–92.
Park JY, Lee SY, Kim HR, Seo MD, Chung KY . Structural mechanism of GPCR-arrestin interaction: recent breakthroughs. Arch Pharm Res 2016; 39: 293–301.
Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS . Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 2000; 275: 17201–17210.
Ahn S, Wei H, Garrison TR, Lefkowitz RJ . Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J Biol Chem 2004; 279: 7807–7811.
Bowen-Pidgeon D, Innamorati G, Sadeghi HM, Birnbaumer M . Arrestin effects on internalization of vasopressin receptors. Mol Pharmacol 2001; 59: 1395–1401.
Ibrahim IA, Kurose H . beta-arrestin-mediated signaling improves the efficacy of therapeutics. J Pharmacol Sci 2012; 118: 408–412.
Whalen EJ, Rajagopal S, Lefkowitz RJ . Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med 2011; 17: 126–139.
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W et al. A stress response pathway regulates DNA damage through beta(2)-adrenoreceptors and beta-arrestin-1. Nature 2011; 477: 349–U129.
Hara MR, Sachs BD, Caron MG, Lefkowitz RJ . Pharmacological blockade of a beta(2)AR-beta-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle 2013; 12: 219–224.
Boularan C, Scott MG, Bourougaa K, Bellal M, Esteve E, Thuret A et al. beta-arrestin 2 oligomerization controls the Mdm2-dependent inhibition of p53. Proc Natl Acad Sci USA 2007; 104: 18061–18066.
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 2011; 477: 349–353.
Zwang Y, Sas-Chen A, Drier Y, Shay T, Avraham R, Lauriola M et al. Two phases of mitogenic signaling unveil roles for p53 and EGR1 in elimination of inconsistent growth signals. Mol Cell 2011; 42: 524–535.
Hongo A, D'Ambrosio C, Miura M, Morrione A, Baserga R . Mutational analysis of the mitogenic and transforming activities of the insulin-like growth factor I receptor. Oncogene 1996; 12: 1231–1238.
Lin FT, Daaka Y, Lefkowitz RJ . Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem 1998; 273: 31640–31643.
Worrall C, Suleymanova N, Crudden C, Trocoli Drakensjo I, Candrea E, Nedelcu D et al. Unbalancing p53/Mdm2/IGF-1R axis by Mdm2 activation restrains the IGF-1-dependent invasive phenotype of skin melanoma. Oncogene 2017; e-pub ahead of print 16 January 2017; doi:10.1038/onc.2016.472.
Castro J, Heiden T, Wang N, Tribukait B . Preparation of cell nuclei from fresh tissues for high-quality DNA flow cytometry. Cytometry 1993; 14: 793–804.
Acknowledgements
We thank Dr Robert J Lefkowitz and Dr Renato Baserga for generously providing reagents and cell lines. Research support was received from the Swedish Research Council, Swedish Cancer Society, The Swedish Childhood Cancer Foundation, Crown Princess Margareta’s Foundation for the Visually Impaired, Welander Finsen Foundation, King Gustaf V Jubilee Foundation, Stockholm Cancer Society, Stockholm County and Karolinska Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Suleymanova, N., Crudden, C., Shibano, T. et al. Functional antagonism of β-arrestin isoforms balance IGF-1R expression and signalling with distinct cancer-related biological outcomes. Oncogene 36, 5734–5744 (2017). https://doi.org/10.1038/onc.2017.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.179
This article is cited by
-
EGFR signaling and pharmacology in oncology revealed with innovative BRET-based biosensors
Communications Biology (2024)
-
IGF-1R is a molecular determinant for response to p53 reactivation therapy in conjunctival melanoma
Oncogene (2022)
-
YAP and endothelin-1 signaling: an emerging alliance in cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Overexpression of β-Arrestins inhibits proliferation and motility in triple negative breast cancer cells
Scientific Reports (2021)