Abstract
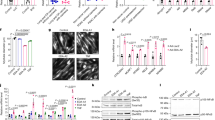
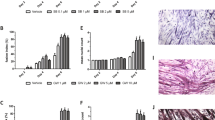
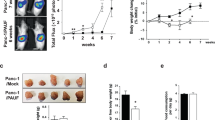
Lung cancer is the leading cause of cancer death worldwide, and is frequently associated with the devastating paraneoplastic syndrome of cachexia. The potent immunomodulatory cytokine interleukin (IL)-6 has been linked with the development of lung cancer as well as cachexia; however, the mechanisms by which IL-6 promotes muscle wasting in lung cancer cachexia are ill-defined. In this study, we report that the gp130F/F knock-in mouse model displaying hyperactivation of the latent transcription factor STAT3 via the common IL-6 cytokine family signalling receptor, gp130, develops cachexia during Kras-driven lung carcinogenesis. Specifically, exacerbated weight loss, early mortality and reduced muscle and adipose tissue mass were features of the gp130F/F:KrasG12D model, but not parental KrasG12D mice in which STAT3 was not hyperactivated. Gene expression profiling of muscle tissue in cachectic gp130F/F:KrasG12D mice revealed the upregulation of IL-6 and STAT3-target genes compared with KrasG12D muscle tissue. These cachectic features of gp130F/F:KrasG12D mice were abrogated upon the genetic normalization of STAT3 activation or ablation of IL-6 in gp130F/F:KrasG12D:Stat3−/+ or gp130F/F:KrasG12D:Il6−/− mice, respectively. Furthermore, protein levels of the soluble IL-6 receptor (sIL-6R), which is the central facilitator of IL-6 trans-signalling, were elevated in cachectic muscle from gp130F/F:KrasG12D mice, and the specific blockade of IL-6 trans-signalling, but not classical signalling, with an anti-IL-6R antibody ameliorated cachexia-related characteristics in gp130F/F:KrasG12D mice. Collectively, these preclinical findings identify trans-signalling via STAT3 as the signalling modality by which IL-6 promotes muscle wasting in lung cancer cachexia, and therefore support the clinical evaluation of the IL-6 trans-signalling/STAT3 axis as a therapeutic target in advanced lung cancer patients presenting with cachexia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004; 90: 1905–1911.
Siegel R, Ma J, Zou Z, Jemal A . Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495.
Del Ferraro C, Grant M, Koczywas M, Dorr-Uyemura LA . Management of anorexia-cachexia in late stage lung cancer patients. J Hosp Palliat Nurs 2012; 14: 397–402.
Ando K, Takahashi F, Kato M, Kaneko N, Doi T, Ohe Y et al. Tocilizumab, a proposed therapy for the cachexia of interleukin6-expressing lung cancer. PLoS One 2014; 9: e102436.
Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R . The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer 1996; 73: 1560–1562.
Songür N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H, Hizel N . Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori 2004; 90: 196–200.
Staal-van den Brekel AJ, Dentener M, Schols AM, Buurman WA, Wouters EF . Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol 1995; 13: 2600–2605.
Ando K, Takahashi F, Motojima S, Nakashima K, Kaneko N, Hoshi K et al. Possible role for tocilizumab, an anti-interleukin-6 receptor antibody, in treating cancer cachexia. J Clin Oncol 2013; 31: e69–e72.
Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR . A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther 2011; 11: 1663–1668.
Tan BH, Ross J, Kaasa S, Skorpen F, Fearon KC . European Palliative Care Research Collaborative. Identification of possible genetic polymorphisms involved in cancer cachexia: a systematic review. J Genet 2011; 90: 165–177.
Ohe Y, Podack ER, Olsen KJ, Miyahara Y, Miura K, Saito H et al. Interleukin-6 cDNA transfected Lewis lung carcinoma cells show unaltered net tumour growth rate but cause weight loss and shortened survival in syngeneic mice. Br J Cancer 1993; 67: 939–944.
Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA . Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 2008; 294: R393–R401.
Strassmann G, Fong M, Kenney JS, Jacob CO . Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 1992; 89: 1681–1684.
Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 2012; 303: E410–E421.
Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest 1996; 97: 244–249.
Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev 2012; 23: 85–97.
Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 2011; 6: e22538.
Puppa MJ, Gao S, Narsale AA, Carson JA . Skeletal muscle glycoprotein 130's role in Lewis lung carcinoma-induced cachexia. FASEB J 2014; 28: 998–1009.
Rose-John S, Scheller J, Elson G, Jones SA . Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol 2006; 80: 227–236.
Brooks GD, McLeod L, Alhayyani S, Miller A, Russell PA, Ferlin W et al. IL6 trans-signaling promotes KRAS-driven lung carcinogenesis. Cancer Res 2016; 76: 866–876.
Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med 2005; 11: 845–852.
Ruwanpura SM, McLeod L, Miller A, Jones J, Bozinovski S, Vlahos R et al. Interleukin-6 promotes pulmonary emphysema associated with apoptosis in mice. Am J Respir Cell Mol Biol 2011; 45: 720–730.
Ruwanpura SM, McLeod L, Miller A, Jones J, Vlahos R, Ramm G et al. Deregulated Stat3 signaling dissociates pulmonary inflammation from emphysema in gp130 mutant mice. Am J Physiol Lung Cell Mol Physiol 2012; 302: L627–L639.
Yu H, Lee H, Herrmann A, Buettner R, Jove R . Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014; 14: 736–746.
Lacroix M, Rousseau F, Guilhot F, Malinge P, Magistrelli G, Herren S et al. Novel insights into interleukin 6 (IL-6) cis- and trans-signaling pathways by differentially manipulating the assembly of the IL-6 signaling complex. J Biol Chem 2015; 290: 26943–26953.
Lissilaa R, Buatois V, Magistrelli G, Williams AS, Jones GW, Herren S et al. Although IL-6 trans-signaling is sufficient to drive local immune responses, classical IL-6 signaling is obligate for the induction of T cell-mediated autoimmunity. J Immunol 2010; 185: 5512–5521.
Ahrendt S, Decker PA, Alawi EA, Zhu YR, Sanchez-Cespedes M, Yang SC et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer 2001; 92: 1525–1530.
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016; 531: 47–52.
Jackson E, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 2001; 15: 3243–3248.
Lesina M, Kurkowski M, Ludes K, Rose-John S, Treiber M, Klöppel G et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011; 19: 456–469.
Gilabert M, Calvo E, Airoldi A, Hamidi T, Moutardier V, Turrini O et al. Pancreatic cancer-induced cachexia is Jak2-dependent in mice. J Cell Physiol 2014; 229: 1437–1443.
Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, van Laethem JL et al. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2014; 20: 2192–2120.
DuPage M, Dooley AL, Jacks T . Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc 2009; 4: 1064–1072.
Kennedy CL, Najdovski M, Tye H, McLeod L, Yu L, Jarnicki A et al. Differential role of MyD88 and Mal/TIRAP in TLR2-mediated gastric tumourigenesis. Oncogene 2013; 33: 2540–2546.
Tye H, Kennedy C, Najdovska M, McLeod L, McCormack W, Hughes N et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell 2012; 22: 466–478.
Acknowledgements
This work was supported by grants awarded by the National Health and Medical Research Council (NHMRC) of Australia and the Lung Cancer Research Foundation, as well as the Operational Infrastructure Support Program by the Victorian Government of Australia. SMR is supported by a NHMRC Post-doctoral Training Fellowship. BJJ is supported by a NHMRC Senior Medical Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Miller, A., McLeod, L., Alhayyani, S. et al. Blockade of the IL-6 trans-signalling/STAT3 axis suppresses cachexia in Kras-induced lung adenocarcinoma. Oncogene 36, 3059–3066 (2017). https://doi.org/10.1038/onc.2016.437
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.437
This article is cited by
-
Growth differentiation factor 11 induces skeletal muscle atrophy via a STAT3-dependent mechanism in pulmonary arterial hypertension
Skeletal Muscle (2022)
-
The critical role of STAT3 in biogenesis of tumor-derived exosomes with potency of inducing cancer cachexia in vitro and in vivo
Oncogene (2022)
-
The interplay of immunology and cachexia in infection and cancer
Nature Reviews Immunology (2022)
-
Epidural adipose tissue-derived mesenchymal stem cell activation induced by lung cancer cells promotes malignancy and EMT of lung cancer
Stem Cell Research & Therapy (2019)
-
Iron-dependent CDK1 activity promotes lung carcinogenesis via activation of the GP130/STAT3 signaling pathway
Cell Death & Disease (2019)