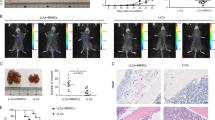
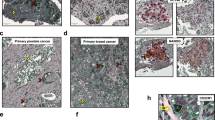
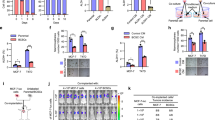
Abstract
Although the role of metastatic cancer stem cells (mCSCs) in tumor progression has been well documented, our study reveals a hitherto unidentified role of tumorigenic intrinsic CSCs (iCSCs) in breast cancer metastasis. We show that unlike highly migratory mCSCs residing in the breast tumor disseminating/peripheral regions, iCSCs populate the inner mass of the tumor and are non-migratory. However iCSCs, via paracrine signaling, induce conversion of non-stem cancer cells to CSCs that (i) are identical to the previously reported mCSCs, and (ii) in contrast to iCSCs, express chemokine receptor, chemokine (C-X-C motif) receptor 4 (CXCR4), which is crucial for their metastatic potential. These mCSCs also demonstrate high in vivo tumorigenicity. Physical non-participation of iCSCs in metastasis is further validated in vivo, where only mCSCs are found to exist in the metastatic sites, lymph nodes and bone marrow, whereas the primary tumor retains both iCSCs and mCSCs. However, iCSCs ensure metastasis since their presence is crucial for deliverance of highly metastatic CXCR4+ mCSCs to the migrating fraction of cells. Cumulatively, these results unveil a novel role of iCSCs in breast cancer metastasis as parental regulators of CXCR4+ mCSCs, and highlight the therapeutic requisite of targeting iCSCs, but not CXCR4+ mCSCs, to restrain breast cancer metastasis from the root by inhibiting the generation of mCSCs from iCSCs. Considering the pivotal role of iCSCs in tumor metastasis, the possibility of metastasis to be a ‘stem cell phenomena’ is suggested.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dick JE . Looking ahead in cancer stem cell research. Nat Biotechnol 2009; 27: 44–46.
Valastyan S, Weinberg RA . Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147: 275–292.
Croker AK, Allan AL . Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med 2008; 12: 374–390.
Oskarsson T, Batlle E, Massague J . Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 2014; 14: 306–321.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007; 1: 313–323.
Baccelli I, Trumpp A . The evolving concept of cancer and metastasis stem cells. J Cell Biol 2012; 198: 281–293.
Wang X, Zhu Y, Ma Y, Wang J, Zhang F, Xia Q et al. The role of cancer stem cells in cancer metastasis: new perspective and progress. Cancer Epidemiol 2013; 37: 60–63.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T . Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer 2005; 5: 744–749.
Singh A, Settleman J . EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010; 29: 4741–4751.
Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010; 140: 62–73.
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009; 69: 1302–1313.
Chaffer CL, Weinberg RA . A perspective on cancer cell metastasis. Science 2011; 331: 1559–1564.
Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 2007; 1: 389–402.
Iliopoulos D, Hirsch HA, Wang G, Struhl K . Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA 2011; 108: 1397–1402.
Yao XH, Ping YF, Chen JH, Xu CP, Chen DL, Zhang R et al. Glioblastoma stem cells produce vascular endothelial growth factor by activation of a G-protein coupled formylpeptide receptor FPR. J Pathol 2008; 215: 369–376.
Chimal-Ramírez GK, Espinoza-Sánchez NA, Fuentes-Pananá EM . Protumor activities of the immune response: insights in the mechanisms of immunological shift, oncotraining, and oncopromotion. J Oncol 2013; 2013: 835956.
Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 2013; 154: 61–74.
Tam WL, Lu H, Buikhuisen J, Soh BS, Lim E, Reinhardt F et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell 2013; 24: 347–364.
Wei W, Hu H, Tan H, Chow LW, Yip AY, Loo WT . Relationship of CD44+CD24-/low breast cancer stem cells and axillary lymph node metastasis. J Transl Med 2012; 10: S6.
Beck B, Blanpain C . Unravelling cancer stem cell potential. Nat Rev Cancer 2013; 13: 727–738.
Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010; 6: 603–615.
Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014; 6: 342–356.
Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A . Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008; 3: e2888.
Lorusso G, Rüegg C . The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 2008; 130: 1091–1103.
Jinushi M, Baghdadi M, Chiba S, Yoshiyama H . Regulation of cancer stem cell activities by tumor-associated macrophages. Am J Cancer Res 2012; 2: 529–539.
Räsänen K, Herlyn M . Paracrine signaling between carcinoma cells and mesenchymal stem cells generates cancer stem cell niche via epithelial-mesenchymal transition. Cancer Discov 2012; 2: 775–777.
Mukherjee D, Zhao J . The Role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res 2013; 3: 46–57.
Park BH, Kook S, Lee S, Jeong JH, Brufsky A, Lee BC . An isoform of C/EBPβ, LIP, regulates expression of the chemokine receptor CXCR4 and modulates breast cancer cell migration. J Biol Chem 2013; 288: 28656–28667.
Ablett MP, O'Brien CS, Sims AH, Farnie G, Clarke RB . A differential role for CXCR4 in the regulation of normal versus malignant breast stem cell activity. Oncotarget 2014; 5: 599–612.
Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T et al. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene 2011; 30: 3833–3845.
Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–56.
Tamamura H, Hori A, Kanzaki N, Hiramatsu K, Mizumoto M, Nakashima H et al. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett 2003; 550: 79–83.
Burger JA, Kipps TJ . CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006; 107: 1761–1767.
Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA 2011; 108: 7950–7955.
Adhikary A, Chakraborty S, Mazumdar M, Ghosh S, Mukherjee S, Manna A et al. Inhibition of Epithelial to Mesenchymal transition by E-cadherin up-regulation via repression of Slug transcription and inhibition of E-cadherin degradation: Dual role of SMAR1 in breast cancer cells. J Biol Chem 2014; 289: 25431–25444.
Lebret SC, Newgreen DF, Thompson EW, Ackland ML . Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res 2007; 9: R19.
Currier N, Solomon SE, Demicco EG, Chang DL, Farago M, Ying H et al. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol Pathol 2005; 33: 726–737.
Wiedswang G, Borgen E, Kåresen R, Qvist H, Janbu J, Kvalheim G et al. Isolated tumor cells in bone marrow three years after diagnosis indisease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res 2004; 10: 5342–5348.
Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D et al. Detection and characterization of circulating tumor cells inblood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res 2009; 11: R59.
Hallab N, Jacobs JJ, Black J . Hypersensitivity to metallic biomaterials: a review of leukocyte migration inhibition assays. Biomaterials 2000; 21: 1301–1314.
Mukherjee S, Mazumdar M, Chakraborty S, Manna A, Saha S, Khan P et al. Curcumin inhibits breast cancer stem cell migration by amplifying E-cadherin/β-catenin negative feed-back loop. Stem Cell Res Ther 2014; 5: 116.
Fillmore CM, Kuperwasser C . Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 2008; 10: R25.
Hossain DM, Panda AK, Manna A, Mohanty S, Bhattacharjee P, Bhattacharyya S et al. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity 2013; 39: 1057–1069.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253–1270.
Acknowledgements
We thank Gaurisankar Sa for his helpful discussions. Authors also acknowledge Uttam K. Ghosh and Ranjan Dutta for technical assistance. This work was supported by research grants from Council of Scientific and Industrial Research (CSIR), Department of Science and Technology (DST) and Department of Biotechnology (DBT), Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Mukherjee, S., Manna, A., Bhattacharjee, P. et al. Non-migratory tumorigenic intrinsic cancer stem cells ensure breast cancer metastasis by generation of CXCR4+ migrating cancer stem cells. Oncogene 35, 4937–4948 (2016). https://doi.org/10.1038/onc.2016.26
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.26
This article is cited by
-
Breast cancer stem cells generate immune-suppressive T regulatory cells by secreting TGFβ to evade immune-elimination
Discover Oncology (2023)
-
A novel lymphatic pattern promotes metastasis of cervical cancer in a hypoxic tumour-associated macrophage-dependent manner
Angiogenesis (2021)
-
Suppression of poised oncogenes by ZMYND8 promotes chemo-sensitization
Cell Death & Disease (2020)
-
A novel role of tumor suppressor ZMYND8 in inducing differentiation of breast cancer cells through its dual-histone binding function
Journal of Biosciences (2020)
-
Aspirin enhances cisplatin sensitivity of resistant non-small cell lung carcinoma stem-like cells by targeting mTOR-Akt axis to repress migration
Scientific Reports (2019)