Abstract
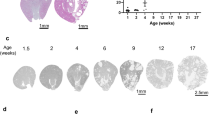
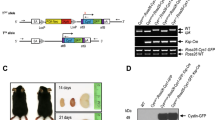
Mutations of the tumor suppressor gene von Hippel–Lindau (VHL) can lead to benign and malignant tumors, including clear-cell renal cell carcinoma (ccRCC). To understand the progression of ccRCC, we generated a novel mouse Vhlh conditional knockout, using Hoxb7-driven Cre that is specific for the collecting ducts and a subset of distal tubules. These mice exhibited wide-spread epithelial disruption and interstitial inflammation as early as 2 months of age with high penetrance. Lesions are cystic, show severe fibrosis and display significant hyperplasia. An abundance of infiltrating macrophages and lymphocytes was detected. Interestingly, the Vhlh mutant lesions could be rescued when Hif-1α, but not Hif-2α, was also knocked out. In addition, administration of a JAK1/2 kinase inhibitor alleviated the Vhlh knockout phenotypes. Taken together, these results suggest that HIF-1α-dependent inflammation and fibrosis may be an early event in the development of ccRCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Frew IJ, Krek W . Pvhl: A multipurpose adaptor protein. Sci Signal 2008; 1: pe30.
Hsu T . Complex cellular functions of the von hippel-lindau tumor suppressor gene: Insights from model organisms. Oncogene 2012; 31: 2247–2257.
Kaelin WG Jr . Treatment of kidney cancer: insights provided by the vhl tumor-suppressor protein. Cancer 2009; 115: 2262–2272.
Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML et al. Identification of the von hippel-lindau disease tumor suppressor gene. Science 1993; 260: 1317–1320.
Maher ER, Neumann HP, Richard S . Von hippel-lindau disease: a clinical and scientific review. Eur J Hum Genet 2011; 19: 617–623.
Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S et al. Silencing of the vhl tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA 1994; 91: 9700–9704.
Kim WY, Kaelin WG . Role of vhl gene mutation in human cancer. J Clin Oncol 2004; 22: 4991–5004.
Kaelin WG Jr . The von hippel-lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 2008; 8: 865–873.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M et al. Hifalpha targeted for vhl-mediated destruction by proline hydroxylation: implications for o2 sensing. Science 2001; 292: 464–468.
Kaelin WG Jr, Ratcliffe PJ . Oxygen sensing by metazoans: the central role of the hif hydroxylase pathway. Mol Cell 2008; 30: 393–402.
Rankin EB, Tomaszewski JE, Haase VH . Renal cyst development in mice with conditional inactivation of the von hippel-lindau tumor suppressor. Cancer Res 2006; 66: 2576–2583.
Frew IJ, Minola A, Georgiev S, Hitz M, Moch H, Richard S et al. Combined vhlh and pten mutation causes genital tract cystadenoma and squamous metaplasia. Mol Cell Biol 2008; 28: 4536–4548.
Ozcan A, Zhai J, Hamilton C, Shen SS, Ro JY, Krishnan B et al. Pax-2 in the diagnosis of primary renal tumors: Immunohistochemical comparison with renal cell carcinoma marker antigen and kidney-specific cadherin. Am J Clin Pathol 2009; 131: 393–404.
Kraus S, Abel PD, Nachtmann C, Linsenmann HJ, Weidner W, Stamp GW et al. Muc1 mucin and trefoil factor 1 protein expression in renal cell carcinoma: correlation with prognosis. Hum Pathol 2002; 33: 60–67.
Khurana KK, Truong LD, Verani RR . Image analysis of proliferating cell nuclear antigen expression and immunohistochemical profiles in renal cell carcinoma associated with acquired cystic kidney disease: Comparison with classic renal cell carcinoma. Mod Pathol 1998; 11: 339–346.
Shen SS, Krishna B, Chirala R, Amato RJ, Truong LD . Kidney-specific cadherin, a specific marker for the distal portion of the nephron and related renal neoplasms. Mod Pathol 2005; 18: 933–940.
Srinivas S, Goldberg MR, Watanabe T, D'Agati V, al-Awqati Q, Costantini F . Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet 1999; 24: 241–251.
Yu J, Carroll TJ, McMahon AP . Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 2002; 129: 5301–5312.
Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 2004; 276: 403–415.
Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM et al. Hif activation identifies early lesions in vhl kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 2002; 1: 459–468.
Kramann R, Dirocco DP, Humphreys BD . Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J Pathol 2013; 231: 273–289.
Nakagawa N, Duffield JS . Myofibroblasts in fibrotic kidneys. Curr Pathobiol Rep 2013; 1: 189–198.
Ries C . Cytokine functions of timp-1. Cell Mol Life Sci 2014; 71: 659–672.
Becker LC . Yin and yang of mcp-1. Circ Res 2005; 96: 812–814.
Perrier S, Darakhshan F, Hajduch E . Il-1 receptor antagonist in metabolic diseases: Dr jekyll or mr hyde? FEBS Lett 2006; 580: 6289–6294.
Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA et al. Preclinical characterization of the selective jak1/2 inhibitor incb018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010; 115: 3109–3117.
Wadleigh M, Tefferi A . Preclinical and clinical activity of atp mimetic jak2 inhibitors. Clin Adv Hematol Oncol 2010; 8: 557–563.
Shay JE, Celest Simon M . Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol 2012; 23: 389–394.
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL et al. Contrasting properties of hypoxia-inducible factor 1 (hif-1) and hif-2 in von hippel-lindau-associated renal cell carcinoma. Mol Cell Biol 2005; 25: 5675–5686.
Martelli F, Ghinassi B, Panetta B, Alfani E, Gatta V, Pancrazzi A et al. Variegation of the phenotype induced by the gata1low mutation in mice of different genetic backgrounds. Blood 2005; 106: 4102–4113.
Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer 2002; 86: 1396–1400.
An J, Rettig MB . Mechanism of von hippel-lindau protein-mediated suppression of nuclear factor kappa b activity. Mol Cell Biol 2005; 25: 7546–7556.
Yang H, Minamishima YA, Yan Q, Schlisio S, Ebert BL, Zhang X et al. Pvhl acts as an adaptor to promote the inhibitory phosphorylation of the nf-kappab agonist card9 by ck2. Mol Cell 2007; 28: 15–27.
Wu KL, Miao H, Khan S . Jak kinases promote invasiveness in vhl-mediated renal cell carcinoma by a suppressor of cytokine signaling-regulated, hif-independent mechanism. Am J Physiol Renal Physiol 2007; 293: F1836–F1846.
Brukamp K, Jim B, Moeller MJ, Haase VH . Hypoxia and podocyte-specific vhlh deletion confer risk of glomerular disease. Am J Physiol Renal Physiol 2007; 293: F1397–F1407.
Steenhard BM, Isom K, Stroganova L St, John PL, Zelenchuk A, Freeburg PB et al. Deletion of von hippel-lindau in glomerular podocytes results in glomerular basement membrane thickening, ectopic subepithelial deposition of collagen {alpha}1{alpha}2{alpha}1(iv), expression of neuroglobin, and proteinuria. Am J Pathol 2010; 177: 84–96.
Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K et al. Stable expression of hif-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 2008; 295: F1023–F1029.
Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B et al. Hypoxia promotes fibrogenesis in vivo via hif-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 2007; 117: 3810–3820.
Schietke RE, Hackenbeck T, Tran M, Gunther R, Klanke B, Warnecke CL et al. Renal tubular hif-2alpha expression requires vhl inactivation and causes fibrosis and cysts. PLoS ONE 2012; 7: e31034.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A . Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009; 30: 1073–1081.
Grivennikov SI, Greten FR, Karin M . Immunity, inflammation, and cancer. Cell 2010; 140: 883–899.
Kraus S, Arber N . Inflammation and colorectal cancer. Curr Opin Pharmacol 2009; 9: 405–410.
Hussain SP, Hofseth LJ, Harris CC . Radical causes of cancer. Nat Rev Cancer 2003; 3: 276–285.
Kundu JK, Surh YJ . Emerging avenues linking inflammation and cancer. Free Radic Biol Med 2012; 52: 2013–2037.
Larkin J, Goh XY, Vetter M, Pickering L, Swanton C . Epigenetic regulation in rcc: opportunities for therapeutic intervention? Nat Rev Urol 2012; 9: 147–155.
Ibragimova I, Maradeo ME, Dulaimi E, Cairns P . Aberrant promoter hypermethylation of pbrm1, bap1, setd2, kdm6a and other chromatin-modifying genes is absent or rare in clear cell rcc. Epigenetics 2013; 8: 486–493.
Hakimi AA, Ostrovnaya I, Reva B, Schultz N, Chen YB, Gonen M et al. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators bap1 and setd2: a report by mskcc and the kirc tcga research network. Clin Cancer Res 2013; 19: 3259–3267.
Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 2010; 463: 360–363.
Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P et al. Exome sequencing identifies frequent mutation of the swi/snf complex gene pbrm1 in renal carcinoma. Nature 2011; 469: 539–542.
Kim N, Hong Y, Kwon D, Yoon S . Somatic mutaome profile in human cancer tissues. Genomics Inform 2013; 11: 239–244.
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C et al. Mutational landscape and significance across 12 major cancer types. Nature 2013; 502: 333–339.
Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J et al. Genome-wide association of hypoxia-inducible factor (hif)-1alpha and hif-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 2009; 284: 16767–16775.
Haase VH, Glickman JN, Socolovsky M, Jaenisch R . Vascular tumors in livers with targeted inactivation of the von hippel-lindau tumor suppressor. Proc Natl Acad Sci USA 2001; 98: 1583–1588.
Tuominen VJ, Ruotoistenmaki S, Viitanen A, Jumppanen M, Isola J . Immunoratio: A publicly available web application for quantitative image analysis of estrogen receptor (er), progesterone receptor (pr), and ki-67. Breast Cancer Res 2010; 12: R56.
Acknowledgements
Paraffin embedding and sectioning were performed at the Immunohistochemistry Core of Boston Medical Center. We thank Richard Near (the Boston University School of Medicine) for technical support and Weining Lu (the Boston University School of Medicine) for a gift of the Hoxb7–Cre–EGFP mouse strain. This work was supported by a grant to TH from the National Institutes of Health, USA (#R01CA109860) and a postdoctoral fellowship to HLB from the National Institutes of Health, USA (#T32HL007501).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pritchett, T., Bader, H., Henderson, J. et al. Conditional inactivation of the mouse von Hippel–Lindau tumor suppressor gene results in wide-spread hyperplastic, inflammatory and fibrotic lesions in the kidney. Oncogene 34, 2631–2639 (2015). https://doi.org/10.1038/onc.2014.197
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.197
This article is cited by
-
HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice
Nature Communications (2020)
-
Active roles of dysfunctional vascular endothelium in fibrosis and cancer
Journal of Biomedical Science (2019)
-
Reduction of hepatic fibrosis by overexpression of von Hippel–Lindau protein in experimental models of chronic liver disease
Scientific Reports (2017)
-
MYC activation cooperates with Vhl and Ink4a/Arf loss to induce clear cell renal cell carcinoma
Nature Communications (2017)
-
Inactivation of the tumor suppressor gene von Hippel-Lindau (VHL) in granulocytes contributes to development of liver hemangiomas in a mouse model
BMC Cancer (2016)