Abstract
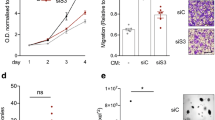
ING proteins have an essential role in the control of a variety of cellular functions whose deregulation is associated with tumor formation and dissemination, such as proliferation, apoptosis, senescence or invasion. Accordingly, loss of function of ING proteins is a frequent event in many types of human tumors. In this report, we have studied the function of ING4, a member of the ING family of tumor suppressors, in the context of normal, non-transformed primary fibroblasts. We show that ING4 negatively regulates cell proliferation in this cell type. The antiproliferative action of ING4 requires its ability to recognize chromatin marks, it is p53-dependent at least in part, and it is lost in an ING4 cancer-associated mutant. Gene expression analysis shows that ING4 regulates the expression and release of soluble factors of the chemokine family. The secretory phenotype regulated by ING4 in primary fibroblasts displays a selective paracrine effect on proliferation, fostering the division of tumor cells, while inhibiting division in primary fibroblasts. Consistently, ING4-expressing fibroblasts promoted tumor growth in vivo in co-injection tumorigenesis assays. Collectively, our results show that ING4 not only can regulate the proliferation of primary non-transformed human fibroblasts, but also orchestrates a secretory phenotype in these cells that promotes tumor cell proliferation in vitro and in vivo. These findings support a critical role for ING4 expression in normal cells in the non-cell-autonomous regulation of tumor growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Aguissa-Toure AH, Wong RP, Li G . The ING family tumor suppressors: from structure to function. Cell Mol Life Sci 2010; 68: 45–54.
Coles AH, Jones SN . The ING gene family in the regulation of cell growth and tumorigenesis. J Cell Physiol 2009; 218: 45–57.
Jafarnejad SM, Li G . Regulation of p53 by ING family members in suppression of tumor initiation and progression. Cancer Metastasis Rev 2012; 31: 55–73.
Ythier D, Larrieu D, Brambilla C, Brambilla E, Pedeux R . The new tumor suppressor genes ING: genomic structure and status in cancer. Int J Cancer 2008; 123: 1483–1490.
Doyon Y, Cayrou C, Ullah M, Landry AJ, Cote V, Selleck W et al. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell 2006; 21: 51–64.
Pena PV, Davrazou F, Shi X, Walter KL, Verkhusha VV, Gozani O et al. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 2006; 442: 100–103.
Shiseki M, Nagashima M, Pedeux RM, Kitahama-Shiseki M, Miura K, Okamura S et al. p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity. Cancer Res 2003; 63: 2373–2378.
Kim S, Chin K, Gray JW, Bishop JM . A screen for genes that suppress loss of contact inhibition: identification of ING4 as a candidate tumor suppressor gene in human cancer. Proc Natl Acad Sci USA 2004; 101: 16251–16256.
Hung T, Binda O, Champagne KS, Kuo AJ, Johnson K, Chang HY et al. ING4 mediates crosstalk between histone H3 K4 trimethylation and H3 acetylation to attenuate cellular transformation. Mol Cell 2009; 33: 248–256.
Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E et al. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature 2004; 428: 328–332.
Shen JC, Unoki M, Ythier D, Duperray A, Varticovski L, Kumamoto K et al. Inhibitor of growth 4 suppresses cell spreading and cell migration by interacting with a novel binding partner, liprin alpha1. Cancer Res 2007; 67: 2552–2558.
Li J, Martinka M, Li G . Role of ING4 in human melanoma cell migration, invasion and patient survival. Carcinogenesis 2008; 29: 1373–1379.
Moreno A, Palacios A, Orgaz JL, Jimenez B, Blanco FJ, Palmero I . Functional impact of cancer-associated mutations in the tumor suppressor protein ING4. Carcinogenesis 2010; 31: 1932–1938.
Tapia C, Zlobec I, Schneider S, Kilic E, Guth U, Bubendorf L et al. Deletion of the inhibitor of growth 4 (ING4) tumor suppressor gene is prevalent in human epidermal growth factor 2 (HER2)-positive breast cancer. Hum Pathol 2011; 42: 983–990.
Coles AH, Gannon H, Cerny A, Kurt-Jones E, Jones SN . Inhibitor of growth-4 promotes IkappaB promoter activation to suppress NF-kappaB signaling and innate immunity. Proc Natl Acad Sci USA 2010; 107: 11423–11428.
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS . The essence of senescence. Genes Dev 2010; 24: 2463–2479.
Collado M, Blasco MA, Serrano M . Cellular senescence in cancer and aging. Cell 2007; 130: 223–233.
Goeman F, Thormeyer D, Abad M, Serrano M, Schmidt O, Palmero I et al. Growth inhibition by the tumor suppressor p33ING1 in immortalized and primary cells: involvement of two silencing domains and effect of Ras. Mol Cell Biol 2005; 25: 422–431.
Abad M, Moreno A, Palacios A, Narita M, Blanco F, Moreno-Bueno G et al. The tumor suppressor ING1 contributes to epigenetic control of cellular senescence. Aging Cell 2011; 10: 158–171.
Pedeux R, Sengupta S, Shen JC, Demidov ON, Saito S, Onogi H et al. ING2 regulates the onset of replicative senescence by induction of p300-dependent p53 acetylation. Mol Cell Biol 2005; 25: 6639–6648.
Menendez C, Abad M, Gomez-Cabello D, Moreno A, Palmero I . ING proteins in cellular senescence. Curr Drug Targets 2009; 10: 406–417.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW . Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88: 593–602.
Wong RP, Lin H, Khosravi S, Piche B, Jafarnejad SM, Chen DW et al. Tumour suppressor ING1b maintains genomic stability upon replication stress. Nucleic Acids Res 2011; 39: 3632–3642.
Larrieu D, Ythier D, Binet R, Brambilla C, Brambilla E, Sengupta S et al. ING2 controls the progression of DNA replication forks to maintain genome stability. EMBO Rep 2009; 10: 1168–1174.
Campisi J, d’Adda di Fagagna F . Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8: 729–740.
Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 2010; 17: 376–387.
Nozell S, Laver T, Moseley D, Nowoslawski L, De Vos M, Atkinson GP et al. The ING4 tumor suppressor attenuates NF-kappaB activity at the promoters of target genes. Mol Cell Biol 2008; 28: 6632–6645.
Erez N, Truitt M, Olson P, Arron ST, Hanahan D . Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 2010; 17: 135–147.
Lazennec G, Richmond A . Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med 2010; 16: 133–144.
Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008; 133: 1006–1018.
Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev 2011; 25: 2125–2136.
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008; 6: 2853–2868.
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008; 133: 1019–1031.
Kuilman T, Peeper DS . Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 2009; 9: 81–94.
Coppe JP, Desprez PY, Krtolica A, Campisi J . The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010; 5: 99–118.
Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J . Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 2001; 98: 12072–12077.
Colla S, Tagliaferri S, Morandi F, Lunghi P, Donofrio G, Martorana D et al. The new tumor-suppressor gene inhibitor of growth family member 4 (ING4) regulates the production of proangiogenic molecules by myeloma cells and suppresses hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: involvement in myeloma-induced angiogenesis. Blood 2007; 110: 4464–4475.
Perkins ND . The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer 2012; 12: 121–132.
Acosta JC, O’Loghlen A, Banito A, Raguz S, Gil J . Control of senescence by CXCR2 and its ligands. Cell Cycle 2008; 7: 2956–2959.
Liu F, Wu S, Ren H, Gu J . Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 2011; 13: 254–262.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD et al. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436: 518–524.
Palmero I, Murga M, Zubiaga A, Serrano M . Activation of ARF by oncogenic stress in mouse fibroblasts is independent of E2F1 and E2F2. Oncogene 2002; 21: 2939–2947.
Gomez-Cabello D, Callejas S, Benguria A, Moreno A, Alonso J, Palmero I . Regulation of the microRNA processor DGCR8 by the tumor suppressor ING1. Cancer Res 2010; 70: 1866–1874.
Acknowledgements
We thank R Gomis, J Massague, G Peters, D Peeper and A Cano for providing cell lines and reagents, V Miguel for help in some experiments and I Peruzza for technical assistance. This work was supported by grants from the Spanish Ministry of Science and Innovation to IP (SAF2009-09031 and SAF2012-32117) and GM-B (SAF2010-20175), and from the Madrid Regional Government (S2010/BMD-2303) to GM-B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Moreno, A., Soleto, I., García-Sanz, P. et al. ING4 regulates a secretory phenotype in primary fibroblasts with dual effects on cell proliferation and tumor growth. Oncogene 33, 1945–1953 (2014). https://doi.org/10.1038/onc.2013.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.145
Keywords
This article is cited by
-
Molecular mechanisms of inhibitor of growth (ING) family members in health and malignancy
Cancer Cell International (2022)
-
Inhibitor of Growth 4 (ING4) is a positive regulator of rRNA synthesis
Scientific Reports (2019)
-
INGs are potential drug targets for cancer
Journal of Cancer Research and Clinical Oncology (2017)
-
Inhibitor of growth-4 is a potential target for cancer therapy
Tumor Biology (2016)
-
Stromal ING1 expression induces a secretory phenotype and correlates with breast cancer patient survival
Molecular Cancer (2015)