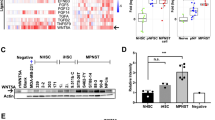
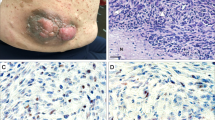
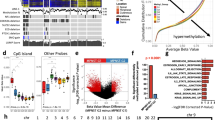
Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) develop sporadically or in the context of neurofibromatosis type 1. Epidermal growth factor receptor (EGFR) overexpression has been implicated in MPNST formation, but its precise role and relevant signaling pathways remain unknown. We found that EGFR overexpression promotes mouse neurofibroma transformation to aggressive MPNST (GEM-PNST). Immunohistochemistry demonstrated phosphorylated STAT3 (Tyr705) in both human MPNST and mouse GEM-PNST. A specific JAK2/STAT3 inhibitor FLLL32 delayed MPNST formation in an MPNST xenograft nude mouse model. STAT3 knockdown by shRNA prevented MPNST formation in vivo. Finally, reducing EGFR activity strongly reduced pSTAT3 in vivo. Thus, an EGFR–STAT3 pathway is necessary for MPNST transformation and establishment of MPNST xenografts growth but not for tumor maintenance. Efficacy of the FLLL32 pharmacological inhibitor in delaying MPNST growth suggests that combination therapies targeting JAK/STAT3 might be useful therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Widemann BC . Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep 2009; 11: 322–328.
Evans D, Baser M, McGaughran J, Sharif S, Howard E, Moran A . Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002; 39: 311–314.
Ferner RE, Gutmann DH . International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res 2002; 62: 1573–1577.
Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM . Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 2005; 65: 205–211.
Ferner RE . Neurofibromatosis 1. Eur J Hum Genet 2007; 15: 131–138.
Carroll SL, Ratner N . How does the Schwann cell lineage form tumors in NF1? Glia 2008; 56: 1590–1605.
Katz D, Lazar A, Lev D . Malignant peripheral nerve sheath tumour (MPNST): the clinical implications of cellular signalling pathways. Expert Rev Mol Med 2009; 11: e30.
Miller SJ, Jessen WJ, Mehta T, Hardiman A, Sites E, Kaiser S et al. Integrative genomic analyses of neurofibromatosis tumours identify SOX9 as a biomarker and survival gene. EMBO Mol Med 2009; 1: 236–248.
Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME et al. Mouse models of tumor development in neurofibromatosis type 1. Science 1999; 286: 2172–2176.
Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF . Mouse tumor model for neurofibromatosis type 1. Science 1999; 286: 2176–2179.
Joseph NM, Mosher JT, Buchstaller J, Snider P, McKeever PE, Lim M et al. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell 2008; 13: 129–140.
Sun D, Tainsky MA, Haddad R . Oncogene mutation survey in MPNST cell lines enhances the dominant role of hyperactive Ras in NF1 associated pro-survival and malignancy. Transl Oncogenomics 2012; 5: 1–7.
Yarden Y . The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001; 37 (Suppl): S3–S8.
DeClue JE, Heffelfinger S, Benvenuto G, Ling B, Li S, Rui W et al. Epidermal growth factor receptor expression in neurofibromatosis type-1 related tumors and NF1 animal models. J Clin Invest 2000; 105: 1–10.
Li H, Velasco-Miguel S, Vass W, Parada L, DeClue J . Epidermal growth factor receptor signaling pathways are associated with tumorigenesis in the Nf1:p53 mouse tumor model. Cancer Res 2002; 62: 4507–4513.
Perry A, Kunz S, Fuller C, Banerjee R, Marley E, Liapis H et al. Differential NF1, p16, and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically similar spindle cell neoplasms. J Neuropathol Exp Neurol 2002; 61: 702–709.
Frohnert PW, Stonecypher MS, Carroll SL . Constitutive activation of the neuregulin-1/ErbB receptor signaling pathway is essential for the proliferation of a neoplastic Schwann cell line. Glia 2003; 43: 104–118.
Keizman D, Issakov J, Meller I, Maimon N, Ish-Shalom M, Sher O et al. Expression and significance of EGFR in malignant peripheral nerve sheath tumor. J Neurooncol 2009; 94: 383–388.
Luetteke N, Phillips H, Qiu T, Copeland N, Earp H, Jenkins N et al. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 1994; 8: 399–413.
Ling B, Wu J, Miller S, Monk K, Shamekh R, Rizvi T et al. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell 2005; 7: 65–75.
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9: 798–809.
Battle TE, Frank DA . The role of STAT3 in apoptosis. Curr Mol Med 2002; 2: 381–392.
Vigneron A, Gamelin E, Coqueret O . The EGFR-STAT3 oncogenic pathway up-regulates the Eme1 endonuclease to reduce DNA damage after topoisomerase I inhibition. Cancer Res 2008; 68: 815–825.
Sherry MM, Reeves A, Wu JK, Cochran BH . STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells 2009; 27: 2383–2392.
Banerjee S, Byrd JN, Gianino SM, Harpstrite SE, Rodriguez FJ, Tuskan RG et al. The neurofibromatosis type 1 tumor suppressor controls cell growth by regulating signal transducer and activator of transcription-3 activity in vitro and in vivo. Cancer Res 2010; 70: 1356–1366.
Lin L, Hutzen B, Zuo M, Ball S, Deangelis S, Foust E et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res 2010; 70: 2445–2454.
Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell 2008; 13: 105–116.
Stemmer-Rachamimov A, Louis D, Nielsen G, Antonescu C, Borowsky A, Bronson R et al. Comparative pathology of nerve sheath tumors in mouse models and humans. Cancer Res 2004; 64: 3718–3724.
Beert E, Brems H, Daniels B, De Wever I, Van Calenbergh F, Schoenaers J et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer 2011; 50: 1021–1032.
Reilly KM, Broman KW, Bronson RT, Tsang S, Loisel DA, Christy ES et al. An imprinted locus epistatically influences Nstr1 and Nstr2 to control resistance to nerve sheath tumors in a neurofibromatosis type 1 mouse model. Cancer Res 2006; 66: 62–68.
Reilly KM, Tuskan RG, Christy E, Loisel DA, Ledger J, Bronson RT et al. Susceptibility to astrocytoma in mice mutant for Nf1 and Trp53 is linked to chromosome 11 and subject to epigenetic effects. Proc Natl Acad Sci USA 2004; 101: 13008–13013.
Walrath JC, Fox K, Truffer E, Gregory Alvord W, Quinones OA, Reilly KM . Chr 19(A/J) modifies tumor resistance in a sex- and parent-of-origin-specific manner. Mamm Genome 2009; 20: 214–223.
Torres KE, Zhu QS, Bill K, Lopez G, Ghadimi MP, Xie X et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res 2011; 17: 3943–3955.
Shao H, Cheng HY, Cook RG, Tweardy DJ . Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res 2003; 63: 3923–3930.
Fukuda A, Wang S, Morris JP, Folias A, Liou A, Kim G et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011; 19: 441–455.
Lesina M, Kurkowski M, Ludes K, Rose-John S, Treiber M, Klöppel G et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011; 19: 456–469.
Guryanova O, Wu Q, Cheng L, Lathia J, Huang Z, Yang J et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell 2011; 19: 498–511.
Marotta L, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker S et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24− stem cell-like breast cancer cells in human tumors. J Clin Invest 2011; 121: 2723–2735.
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 2007; 67: 9066–9076.
Miller SJ, Rangwala F, Williams J, Ackerman P, Kong S, Jegga AG et al. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res 2006; 66: 2584–2591.
Frahm S, Mautner VF, Brems H, Legius E, Debiec-Rychter M, Friedrich RE et al. Genetic and phenotypic characterization of tumor cells derived from malignant peripheral nerve sheath tumors of neurofibromatosis type 1 patients. Neurobiol Dis 2004; 16: 85–91.
Rosenbaum T, Rosenbaum C, Winner U, Muller HW, Lenard HG, Hanemann CO . Long-term culture and characterization of human neurofibroma-derived Schwann cells. J Neurosci Res 2000; 61: 524–532.
Acknowledgements
We thank Dr Andre Bernards (Massachusetts General Hospital) and Drs Jiayuh Lin, Pui-Kai Li and Gregory B Lesinski (Ohio State University) for helpful discussions. This work was supported by the National Institutes of Health (R01 NS28840 and P50 NS057531 to NR), and Department of Defense New Investigator Award (NF100053) and an Ohio State University Comprehensive Cancer Center Pelotonia Idea Grant (to JW). The American Cancer Society (IRG-67-003-44) supported JRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, J., Patmore, D., Jousma, E. et al. EGFR–STAT3 signaling promotes formation of malignant peripheral nerve sheath tumors. Oncogene 33, 173–180 (2014). https://doi.org/10.1038/onc.2012.579
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.579
Keywords
This article is cited by
-
Comprehensive pan-cancer analysis of STAT3 as a prognostic and immunological biomarker
Scientific Reports (2023)
-
The therapeutic potential of neurofibromin signaling pathways and binding partners
Communications Biology (2023)
-
A clinicopathologic study of malignancy in VCP-associated multisystem proteinopathy
Orphanet Journal of Rare Diseases (2022)
-
Matrix protein Tenascin-C promotes kidney fibrosis via STAT3 activation in response to tubular injury
Cell Death & Disease (2022)
-
Efficacy of MEK inhibition in a recurrent malignant peripheral nerve sheath tumor
npj Precision Oncology (2021)