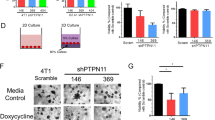
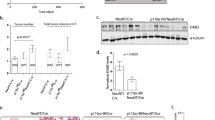
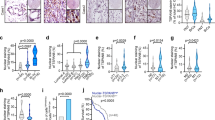
Abstract
The ShcA adapter protein transmits activating signals downstream of receptor and cytoplasmic tyrosine kinases through the establishment of phosphotyrosine-dependent complexes. In this regard, ShcA possesses both a phosphotyrosine-binding domain (PTB) and Src homology 2 domain (SH2), which bind phosphotyrosine residues in a sequence-specific manner. Although the majority of receptor tyrosine kinases expressed in breast cancer cells bind the PTB domain, very little is known regarding the biological importance of SH2-driven ShcA signaling during mammary tumorigenesis. To address this, we employed transgenic mice expressing a mutant ShcA allele harboring a non-functional SH2 domain (ShcR397K) under the transcriptional control of the endogenous ShcA promoter. Using transplantation approaches, we demonstrate that SH2-dependent ShcA signaling within the mammary epithelial compartment is essential for breast tumor outgrowth, survival and the development of lung metastases. We further show that the ShcA SH2 domain activates the AKT pathway, potentially through a novel SH2-mediated complex between ShcA, 14-3-3ζ and the p85 regulatory subunit of phosphatidylinositol 3 (PI3′) kinase. This study is the first to demonstrate that the SH2 domain of ShcA is critical for tumor survival during mammary tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frackelton Jr AR, Lu L, Davol PA, Bagdasaryan R, Hafer LJ, Sgroi DC . p66 Shc and tyrosine-phosphorylated Shc in primary breast tumors identify patients likely to relapse despite tamoxifen therapy. Breast Cancer Res 2006; 8: R73.
Davol PA, Bagdasaryan R, Elfenbein GJ, Maizel AL, Frackelton Jr AR . Shc proteins are strong, independent prognostic markers for both node-negative and node-positive primary breast cancer. Cancer Res 2003; 63: 6772–6783.
Ursini-Siegel J, Cory S, Zuo D, Hardy WR, Rexhepaj E, Lam S et al. Receptor tyrosine kinase signaling favors a protumorigenic state in breast cancer cells by inhibiting the adaptive immune response. Cancer Res 2010; 70: 7776–7787.
Ursini-Siegel J, Hardy WR, Zuo D, Lam SH, Sanguin-Gendreau V, Cardiff RD et al. ShcA signalling is essential for tumour progression in mouse models of human breast cancer. EMBO J 2008; 27: 910–920.
Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995; 10: 1813–1821.
Batzer AG, Blaikie P, Nelson K, Schlessinger J, Margolis B . The phosphotyrosine interaction domain of Shc binds an LXNPXY motif on the epidermal growth factor receptor. Mol Cell Biol 1995; 15: 4403–4409.
Dankort DL, Wang Z, Blackmore V, Moran MF, Muller WJ . Distinct tyrosine autophosphorylation sites negatively and positively modulate neu-mediated transformation. Mol Cell Biol 1997; 17: 5410–5425.
Gillgrass A, Cardiff RD, Sharan N, Kannan S, Muller WJ . Epidermal growth factor receptor-dependent activation of Gab1 is involved in ErbB-2-mediated mammary tumor progression. Oncogene 2003; 22: 9151–9155.
Ishiguro Y, Iwashita T, Murakami H, Asai N, Iida K, Goto H et al. The role of amino acids surrounding tyrosine 1062 in ret in specific binding of the shc phosphotyrosine-binding domain. Endocrinology 1999; 140: 3992–3998.
Jones RA, Campbell CI, Wood GA, Petrik JJ, Moorehead RA . Reversibility and recurrence of IGF-IR-induced mammary tumors. Oncogene 2009; 28: 2152–2162.
Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P . Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 1988; 54: 105–115.
Vijapurkar U, Cheng K, Koland JG . Mutation of a Shc binding site tyrosine residue in ErbB3/HER3 blocks heregulin-dependent activation of mitogen-activated protein kinase. J Biol Chem 1998; 273: 20996–21002.
Zhang Z, Kumar R, Santen RJ, Song RX . The role of adapter protein Shc in estrogen non-genomic action. Steroids 2004; 69: 523–529.
Guy CT, Cardiff RD, Muller WJ . Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992; 12: 954–961.
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 2003; 163: 2113–2126.
Webster MA, Hutchinson JN, Rauh MJ, Muthuswamy SK, Anton M, Tortorice CG et al. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol 1998; 18: 2344–2359.
Rauh MJ, Blackmore V, Andrechek ER, Tortorice CG, Daly R, Lai VK et al. Accelerated mammary tumor development in mutant polyomavirus middle T transgenic mice expressing elevated levels of either the Shc or Grb2 adapter protein. Mol Cell Biol 1999; 19: 8169–8179.
Hardy WR, Li L, Wang Z, Sedy J, Fawcett J, Frank E et al. Combinatorial ShcA docking interactions support diversity in tissue morphogenesis. Science 2007; 317: 251–256.
Barry EF, Felquer FA, Powell JA, Biggs L, Stomski FC, Urbani A et al. 14-3-3:Shc scaffolds integrate phosphoserine and phosphotyrosine signaling to regulate phosphatidylinositol 3-kinase activation and cell survival. J Biol Chem 2009; 284: 12080–12090.
Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature 1992; 360: 689–692.
van der Geer P, Wiley S, Gish GD, Pawson T . The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol 1996; 6: 1435–1444.
Ling C, Zuo D, Xue B, Muthuswamy S, Muller WJ . A novel role for 14-3-3sigma in regulating epithelial cell polarity. Genes Dev 2010; 24: 947–956.
Orlando FA, Brown KD . Unraveling breast cancer heterogeneity through transcriptomic and epigenomic analysis. Ann Surg Oncol 2009; 16: 2270–2279.
Su CH, Zhao R, Zhang F, Qu C, Chen B, Feng YH et al. 14-3-3sigma exerts tumor-suppressor activity mediated by regulation of COP1 stability. Cancer Res 2011; 71: 884–894.
Hodgson JG, Malek T, Bornstein S, Hariono S, Ginzinger DG, Muller WJ et al. Copy number aberrations in mouse breast tumors reveal loci and genes important in tumorigenic receptor tyrosine kinase signaling. Cancer Res 2005; 65: 9695–9704.
Neal CL, Xu J, Li P, Mori S, Yang J, Neal NN et al. Overexpression of 14-3-3zeta in cancer cells activates PI3K via binding the p85 regulatory subunit. Oncogene 2011.
Gotoh N, Tojo A, Shibuya M . A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J 1996; 15: 6197–6204.
Patrussi L, Savino MT, Pellegrini M, Paccani SR, Migliaccio E, Plyte S et al. Cooperation and selectivity of the two Grb2 binding sites of p52Shc in T-cell antigen receptor signaling to Ras family GTPases and Myc-dependent survival. Oncogene 2005; 24: 2218–2228.
Gu H, Maeda H, Moon JJ, Lord JD, Yoakim M, Nelson BH et al. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol 2000; 20: 7109–7120.
Bone H, Welham MJ . Shc associates with the IL-3 receptor beta subunit, SHIP and Gab2 following IL-3 stimulation. Contribution of Shc PTB and SH2 domains. Cell Signal 2000; 12: 183–194.
Campbell KS, Ogris E, Burke B, Su W, Auger KR, Druker BJ et al. Polyoma middle tumor antigen interacts with SHC protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc Natl Acad Sci USA 1994; 91: 6344–6348.
Cook RS, Garrett JT, Sanchez V, Stanford JC, Young C, Chakrabarty A et al. ErbB3 ablation impairs PI3K/Akt-dependent mammary tumorigenesis. Cancer Res 2011; 71: 3941–3951.
Morrison DK . The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 2009; 19: 16–23.
Backer JM, Myers Jr MG, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J 1992; 11: 3469–3479.
Zhou MM, Harlan JE, Wade WS, Crosby S, Ravichandran KS, Burakoff SJ et al. Binding affinities of tyrosine-phosphorylated peptides to the COOH-terminal SH2 and NH2-terminal phosphotyrosine binding domains of Shc. J Biol Chem 1995; 270: 31119–31123.
Telles E, Hosing AS, Kundu ST, Venkatraman P, Dalal SN . A novel pocket in 14-3-3epsilon is required to mediate specific complex formation with cdc25C and to inhibit cell cycle progression upon activation of checkpoint pathways. Exp Cell Res 2009; 315: 1448–1457.
Wang B, Liu K, Lin FT, Lin WC . A role for 14-3-3 tau in E2F1 stabilization and DNA damage-induced apoptosis. J Biol Chem 2004; 279: 54140–54152.
Komiya Y, Kurabe N, Katagiri K, Ogawa M, Sugiyama A, Kawasaki Y et al. A novel binding factor of 14-3-3beta functions as a transcriptional repressor and promotes anchorage-independent growth, tumorigenicity, and metastasis. J Biol Chem 2008; 283: 18753–18764.
Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B et al. 14-3-3zeta Cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell 2009; 16: 195–207.
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 2009; 69: 3425–3432.
George R, Schuller AC, Harris R, Ladbury JE . A phosphorylation-dependent gating mechanism controls the SH2 domain interactions of the Shc adaptor protein. J Mol Biol 2008; 377: 740–747.
Dankort D, Maslikowski B, Warner N, Kanno N, Kim H, Wang Z et al. Grb2 and Shc adapter proteins play distinct roles in Neu (ErbB-2)-induced mammary tumorigenesis: implications for human breast cancer. Mol Cell Biol 2001; 21: 1540–1551.
Dilworth SM, Griffin BE . Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci USA 1982; 79: 1059–1063.
Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ . Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res 2009; 69: 6823–6830.
Ursini-Siegel J, Rajput AB, Lu H, Sanguin-Gendreau V, Zuo D, Papavasiliou V et al. Elevated expression of DecR1 impairs ErbB2/Neu-induced mammary tumor development. Mol Cell Biol 2007; 27: 6361–6371.
Acknowledgements
We thank Dr Peter Siegel for critical reading of this manuscript and Vasilios Papavasiliou for assisting with the injection studies. This work was supported by CBCRA, CIHR (MOP-89751) and Terry Fox team (020002) grants to WJM, a CIHR operating grant to JU-S (MOP-111143) and CIHR (MOP6849) and Terry Fox Foundation/CIHR (TFF105268) grants to TP JU-S is the recipient of a CIHR New Investigator Salary Support award. LP is supported by a CIHR/FRSQ training grant in cancer research of the McGill Integrated Cancer Research Training Program (MICRTP). WJM is supported by a CRC chair in molecular oncology. TP is a CIHR distinguished scientist.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Ursini-Siegel, J., Hardy, W., Zheng, Y. et al. The ShcA SH2 domain engages a 14-3-3/PI3′K signaling complex and promotes breast cancer cell survival. Oncogene 31, 5038–5044 (2012). https://doi.org/10.1038/onc.2012.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.4
Keywords
This article is cited by
-
VEPH1 expression decreases vascularisation in ovarian cancer xenografts and inhibits VEGFA and IL8 expression through inhibition of AKT activation
British Journal of Cancer (2017)
-
Interaction with Shc prevents aberrant Erk activation in the absence of extracellular stimuli
Nature Structural & Molecular Biology (2013)