Abstract
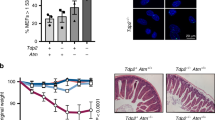
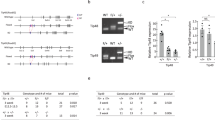
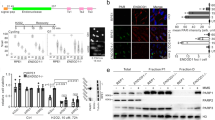
Wild-type p53-induced phosphatase 1 (WIP1) is a serine/threonine phosphatase that dephosphorylates proteins in the ataxia telangiectasia mutated (ATM)-initiated DNA damage response pathway. WIP1 may have a homeostatic role in ATM signaling by returning the cell to a normal pre-stress state following completion of DNA repair. To better understand the effects of WIP1 on ATM signaling, we crossed Atm-deficient mice to Wip1-deficient mice and characterized phenotypes of the double knockout progeny. We hypothesized that the absence of Wip1 might rescue Atm deficiency phenotypes. Atm null mice, like ATM-deficient humans with the inherited syndrome ataxia telangiectasia, exhibit radiation sensitivity, fertility defects, and are T-cell lymphoma prone. Most double knockout mice were largely protected from lymphoma development and had a greatly extended lifespan compared with Atm null mice. Double knockout mice had increased p53 and H2AX phosphorylation and p21 expression compared with their Atm null counterparts, indicating enhanced p53 and DNA damage responses. Additionally, double knockout splenocytes displayed reduced chromosomal instability compared with Atm null mice. Finally, doubly null mice were partially rescued from gametogenesis defects observed in Atm null mice. These results indicate that inhibition of WIP1 may represent a useful strategy for cancer treatment in general and A-T patients in particular.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Abraham RT . (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196.
Appella E, Anderson CW . (2001). Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268: 2764–2772.
Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F et al. (1996). Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171.
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K et al. (2005). DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434: 864–870.
Belova GI, Demidov ON, Fornace AJ, Bulavin DV . (2005). Chemical inhibition of Wip1 phosphatase contributes to suppression of tumorigenesis. Cancer Biol Ther 4: 1154–1158.
Borghesani PR, Alt FW, Bottaro A, Davidson L, Aksoy S, Rathbun GA et al. (2000). Abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc Natl Acad Sci USA 97: 3336–3341.
Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA et al. (2002). Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 31: 210–215.
Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW et al. (2004). Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet 36: 343–350.
Castellino RC, De Bortoli M, Lu X, Moon SH, Nguyen TA, Shepard MA et al. (2008). Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J Neurooncol 86: 245–256.
Choi J, Nannenga B, Demidov ON, Bulavin DV, Cooney A, Brayton C et al. (2002). Mice deficient for the wild-type p53-induced phosphatase gene (Wip1) exhibit defects in reproductive organs, immune function, and cell cycle control. Mol Cell Biol 22: 1094–1105.
Chun HH, Gatti RA . (2004). Ataxia-telangiectasia, an evolving phenotype. DNA Repair (Amst) 3: 1187–1196.
Demidov ON, Timofeev O, Lwin HN, Kek C, Appella E, Bulavin DV . (2007). Wip1 phosphatase regulates p53-dependent apoptosis of stem cells and tumorigenesis in the mouse intestine. Cell Stem Cell 1: 180–190.
Ehrbrecht A, Muller U, Wolter M, Hoischen A, Koch A, Radlwimmer B et al. (2006). Comprehensive genomic analysis of desmoplastic medulloblastomas: identification of novel amplified genes and separate evaluation of the different histological components. J Pathol 208: 554–563.
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 19: 817–825.
Elson A, Wang Y, Daugherty CJ, Morton CC, Zhou F, Campos-Torres J et al. (1996). Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci USA 93: 13084–13089.
Fiscella M, Zhang HL, Fan SJ, Sakaguchi K, Shen SF, Mercer WE et al. (1997). Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci USA 94: 6048–6053.
Fujimoto H, Onishi N, Kato N, Takekawa M, Xu X, Kosugi A et al. (2006). Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1 phosphatase. Cell Death Different 13: 1170–1180.
Gurley KE, Kemp CJ . (2001). Synthetic lethality between mutation in Atm and DNA-PK(cs) during murine embryogenesis. Curr Biol 11: 191–194.
Halazonetis TD, Gorgoulis VG, Bartek J . (2008). An oncogene-induced DNA damage model for cancer development. Science 319: 1352–1355.
Harrison M, Li J, Degenhardt Y, Hoey T, Powers S . (2004). Wip1-deficient mice are resistant to common cancer genes. Trends Mol Med 10: 359–361.
Herzog KH, Chong MJ, Kapsetaki M, Morgan JI, McKinnon PJ . (1998). Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science 280: 1089–1091.
Hirasawa A, Saito-Ohara F, Inoue J, Aoki D, Susumu N, Yokoyama T et al. (2003). Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin Cancer Res 9: 1995–2004.
Jiang H, Reinhardt HC, Bartkova J, Tommiska J, Blomqvist C, Nevanlinna H et al. (2009). The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev 23: 1895–1909.
Lavin MF . (2008). Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 9: 759–769.
Le Guezennec X, Bulavin DV . (2009). WIP1 phosphatase at the crossroads of cancer and aging. Trends Biochem Sci 35: 109–114.
Li J, Yang Y, Peng Y, Austin RJ, van Eyndhoven G, Nguyen KCQ et al. (2002). Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet 31: 133–134.
Li M, Fang X, Baker DJ, Guo L, Gao X, Wei Z et al. (2010). The ATM-p53 pathway suppresses aneuploidy-induced tumorigenesis. Proc Natl Acad Sci USA 107: 14188–14193.
Loukopoulos P, Shibata T, Katoh H, Kokubu A, Sakamoto M, Yamazaki K et al. (2007). Genome-wide array-based comparative genomic hybridization analysis of pancreatic adenocarcinoma: Identification of genetic indicators that predict patient outcome. Cancer Sci 98: 392–400.
Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA . (2007). The Wip1 Phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell 12: 342–354.
Lu X, Nannenga B, Donehower LA . (2005a). PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev 19: 1162–1174.
Lu XB, Nguyen TA, Donehower LA . (2005b). Reversal of the ATM/ATR-mediated DNA damage response by the oncogenic phosphatase PPM1D. Cell Cycle 4: 1060–1064.
Lu X, Nguyen TA, Moon SH, Darlington Y, Sommer M, Donehower LA . (2008). The type 2C phosphatase Wip1: an oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev 27: 123–135.
Macurek L, Lindqvist A, Voets O, Kool J, Vos HR, Medema RH . (2010). Wip1 phosphatase is associated with chromatin and dephosphorylates gammaH2AX to promote checkpoint inhibition. Oncogene 29: 2281–2291.
Moon SH, Lin L, Zhang X, Nguyen TA, Darlington Y, Waldman AS et al. (2010). Wildtype p53-induced phosphatase 1 dephosphorylates histone variant {gamma}-H2AX and suppresses DNA double. J Biol Chem 285: 12935–12947.
Moretti P, Bouwknecht JA, Teague R, PaylorR, Zoghbi HY . (2005). Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet 14: 205–220.
Nannenga B, Lu X, Dumble M, Van Maanen M, Nguyen TA, Sutton R et al. (2006). Augmented cancer resistance and DNA damage response phenotypes in PPM1D null mice. Mol Carcinog 45: 594–604.
Rao PH, Cigudosa JC, Ning Y, Calasanz MJ, Iida S, Tagawa S et al. (1998). Multicolor spectral karyotyping identifies new recurring breakpoints and translocations in multiple myeloma. Blood 92: 1743–1748.
Rayter S, Elliott R, Travers J, Rowlands MG, Richardson TB, Boxall K et al. (2008). A chemical inhibitor of PPM1D that selectively kills cells overexpressing PPM1D. Oncogene 27: 1036–1044.
Rotman G, Shiloh Y . (1998). ATM: from gene to function. Hum Mol Genet 7: 1555–1563.
Saito-Ohara F, Imoto I, Inoue J, Hosoi H, Nakagawara A, Sugimoto T et al. (2003). PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res 63: 1876–1883.
Savitsky K, Sfez S, Tagle DA, Ziv Y, Sartiel A, Collins FS et al. (1995). The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet 4: 2025–2032.
Sedgwick RP, Boder E . (1991). Ataxia-Telangiectasia. In: deJong JMBE (ed). Hereditary Neuropathies and Spinocerebellar Atrophies. Elsevier: New York, pp 347–423 (Vinken PJ, Bruyn GW, Klawans HL, eds. Handbook of Clinical Neurology, Vol 16).
Shen KC, Heng H, Wang Y, Lu S, Liu G, Deng CX et al. (2005). ATM and p21 cooperate to suppress aneuploidy and subsequent tumor development. Cancer Res 65: 8747–8753.
Shiloh Y . (2003). ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168.
Shreeram S, Demidov ON, Hee WK, Yamaguchi H, Onishi N, Kek C et al. (2006a). Wip1 phosphatase modulates ATM-dependent signaling pathways. Mol Cell 23: 757–764.
Shreeram S, Hee WK, Demidov ON, Kek C, Yamaguchi H, Fornace AJ et al. (2006b). Regulation of ATM/p53-dependent suppression of myc-induced lymphomas by Wip1 phosphatase. J Exp Med 203: 2793–2799.
Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H et al. (2000). p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 19: 6517–6526.
Tan DS, Lambros MB, Rayter S, Natrajan R, Vatcheva R, Gao Q et al. (2009). PPM1D is a potential therapeutic target in ovarian clear cell carcinomas. Clin Cancer Res 15: 2269–2280.
Westphal CH, Rowan S, Schmaltz C, Elson A, Fisher DE, Leder P . (1997). Atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat Genet 16: 397–401.
Williamson CT, Muzik H, Turhan AG, Zamo A, O'Connor MJ, Bebb DG et al. (2010). ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol Cancer Ther 9: 347–357.
Xia Y, Ongusaha P, Lee SW, Liou YC . (2009). Loss of Wip1 sensitizes cells to stress- and DNA damage-induced apoptosis. J Biol Chem 284: 17428–17437.
Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D . (1996). Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev 10: 2411–2422.
Yamaguchi H, Durell SR, Feng HQ, Bai YW, Anderson CW, Appella E . (2006). Development of a substrate-based cyclic phosphopeptide inhibitor of protein phosphatase 2C delta, Wip1. Biochemistry 45: 13193–13202.
Yoda A, Toyoshima K, Watanabe Y, Onishi N, Hazaka Y, Tsukuda Y et al. (2008). Arsenic trioxide augments Chk2/p53-mediated apoptosis by inhibiting oncogenic Wip1 phosphatase. J Biol Chem 283: 18969–18979.
Acknowledgements
We thank Corrine Spencer and Yi-Jue Zhao for technical assistance. This work was supported by NIH grants (R01 CA100420) to LAD and (R01 CA136549) to XL, and a DOD Breast Cancer Research Program Predoctoral Traineeship Award (BC050781) to T-AN
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Darlington, Y., Nguyen, TA., Moon, SH. et al. Absence of Wip1 partially rescues Atm deficiency phenotypes in mice. Oncogene 31, 1155–1165 (2012). https://doi.org/10.1038/onc.2011.303
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.303
Keywords
This article is cited by
-
Dynamic recruitment of UFM1-specific peptidase 2 to the DNA double-strand breaks regulated by WIP1
Genome Instability & Disease (2022)
-
ATM signalling and cancer
Oncogene (2014)
-
The ATM protein kinase: regulating the cellular response to genotoxic stress, and more
Nature Reviews Molecular Cell Biology (2013)