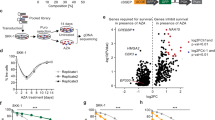
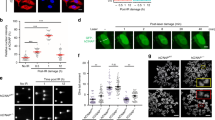
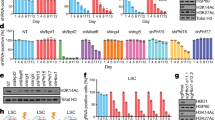
Abstract
The chromatin-associated AAA+ ATPases Tip48 and Tip49 are the core components of various complexes implicated in diverse nuclear events such as DNA repair and gene regulation. Although they are frequently overexpressed in many human cancers, their functional significance remains unclear. Here, we show that loss of Tip49 triggered p53-dependent apoptosis and inhibited leukemia development in vivo. To examine the impact of chemical inhibition of this complex on leukemia, we have developed the novel compound DS-4950, which interferes with the ATPase activity of the Tip48/49. Administration of DS-4950 was well-tolerated in healthy mice, and the drug effectively reduced tumor burden and improved survival. We also provide evidence that the dependency on Tip48/49 is widely conserved in non-hematologic malignancies with wild type p53. These results demonstrated that the Tip48/49 ATPases are functionally necessary and therapeutically targetable for the treatment of human cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Clapier CR, Iwasa J, Cairns BR, Peterson CL. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat Rev Mol Cell Bio. 2017;18:407–22.
Martens JA, Winston F. Recent advances in understanding chromatin remodeling by Swi/Snf complexes. Curr Opin Genet Dev. 2003;13:136–42.
Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, part I: covalent histone modifications. Trends Mol Med. 2007;13:363–72.
Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, part II: ATP-dependent chromatin remodeling. Trends Mol Med. 2007;13:373–80.
Hohmann AF, Vakoc CR. A rationale to target the SWI/SNF complex for cancer therapy. Trends Genet. 2014;30:356–63.
Kadoch C, Crabtree GR. Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Sci Adv. 2015;1:e1500447.
Willhoft O, Wigley DB. INO80 and SWR1 complexes: the non-identical twins of chromatin remodelling. Curr Opin Struc Biol. 2020;61:50–8.
Poli J, Gasser SM, Papamichos-Chronakis M. The INO80 remodeller in transcription, replication and repair. Philos Trans R Soc B Biol Sci. 2017;372:20160290.
Dauden MI, López-Perrote A, Llorca O. RUVBL1–RUVBL2 AAA-ATPase: a versatile scaffold for multiple complexes and functions. Curr Opin Struc Biol. 2021;67:78–85.
Matias PM, Gorynia S, Donner P, Carrondo MA. Crystal structure of the human AAA+ protein RuvBL1*. J Biol Chem. 2006;281:38918–29.
Gorynia S, Bandeiras TM, Pinho FG, McVey CE, Vonrhein C, Round A, et al. Structural and functional insights into a dodecameric molecular machine – The RuvBL1/RuvBL2 complex. J Struct Biol. 2011;176:279–91.
Zhou CY, Stoddard CI, Johnston JB, Trnka MJ, Echeverria I, Palovcak E, et al. Regulation of Rvb1/Rvb2 by a domain within the INO80 chromatin remodeling complex implicates the yeast Rvbs as protein assembly chaperones. Cell Reports. 2017;19:2033–44.
Houry WA, Bertrand E, Coulombe B. The PAQosome, an R2TP-based chaperone for quaternary structure formation. Trends Biochem Sci. 2018;43:4–9.
Yamagata K, Shino M, Aikawa Y, Fujita S, Kitabayashi I. Tip60 activates Hoxa9 and Meis1 expression through acetylation of H2A.Z, promoting MLL-AF10 and MLL-ENL acute myeloid leukemia. Leukemia. 2021;35:2840–53.
Assimon VA, Tang Y, Vargas JD, Lee GJ, Wu ZY, Lou K, et al. CB-6644 Is A Selective Inhibitor of the RUVBL1/2 complex with anticancer activity. ACS chemical biology. 2019;14:236–44.
Yenerall P, Das AK, Wang S, Kollipara RK, Li LS, Villalobos P, et al. RUVBL1/RUVBL2 ATPase activity drives PAQosome maturation, DNA replication and radioresistance in lung cancer. Cell chemical biology. 2020;27:105–21.e14.
Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:R60.
Puri T, Wendler P, Sigala B, Saibil H, Tsaneva IR. Dodecameric structure and ATPase activity of the human TIP48/TIP49 complex. J Mol Biol. 2007;366:179–92.
Mao Y-Q, Houry WA. The role of pontin and reptin in cellular physiology and cancer etiology. Frontiers Mol Biosci. 2017;4:58.
Sun Y, Jiang X, Price BD. Tip60: connecting chromatin to DNA damage signaling. Cell Cycle. 2010;9:930–6.
Jha S, Shibata E, Dutta A. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol Cell Biol. 2008;28:2690–700.
Ikura T, Tashiro S, Kakino A, Shima H, Jacob N, Amunugama R, et al. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol Cell Biol. 2007;27:7028–40.
Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–73.
Armenteros-Monterroso E, Zhao L, Gasparoli L, Brooks T, Pearce K, Mansour MR, et al. The AAA+ATPase RUVBL2 is essential for the oncogenic function of c-MYB in acute myeloid leukemia. Leukemia. 2019;33:2817–29.
Osaki H, Walf-Vorderwülbecke V, Mangolini M, Zhao L, Horton SJ, Morrone G, et al. The AAA+ ATPase RUVBL2 is a critical mediator of MLL-AF9 oncogenesis. Leukemia. 2013;27:1461–8.
Breig O, Bras S, Soria NM, Osman D, Heidenreich O, Haenlin M, et al. Pontin is a critical regulator for AML1-ETO-induced leukemia. Leukemia. 2014;28:1271–9.
Jacoby MA, Pizarro REDJ, Shao J, Koboldt DC, Fulton RS, Zhou G, et al. The DNA double-strand break response is abnormal in myeloblasts from patients with therapy-related acute myeloid leukemia. Leukemia. 2014;28:1242–51.
Esposito MT, So CWE. DNA damage accumulation and repair defects in acute myeloid leukemia: implications for pathogenesis, disease progression, and chemotherapy resistance. Chromosoma. 2014;123:545–61.
Shieh S-Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–34.
Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh S-Y, et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Gene Dev. 1999;13:152–7.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. New Engl J Med. 2016;374:2209–21.
Acknowledgements
We thank Dr. Toshio Imai at the Central Animal Division for maintaining animals, Dr. Masamichi Ishiai at The Central Radioisotope Division for advice on radioisotope use, Rie Sawado and Yukiko Aikawa for technical help, and Drs. Takahiro Ito and Myriam Y Hsu for comments on the manuscript. This work was supported by AMED (JP19cm0106141 and JP21cm010617), JSPS KAKENHI (JP22H03103), the Japan Science and Technology Agency (grant JPMJMS2022-16) and Project for Cancer Research and Therapeutic Evolution (P-CREATE).
Author information
Authors and Affiliations
Contributions
AH designed the study, performed all experiments, analyzed the data, and wrote the manuscript. ET-I generated Tip48 and Tip49 cKO mice. YY performed western blotting, immunofluorescence, and bioinformatics analysis. SF and YM performed part of drug treatment analysis. RM provided the compound. KY, TK and HS contributed essential reagents. IK conceived and supervised the project. All authors read and edited manuscript.
Corresponding authors
Ethics declarations
Competing interests
RM is an employee of Daiichi Sankyo, Co., Ltd. All other authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hattori, A., Takamatsu-Ichihara, E., Yamamoto, Y. et al. Genetic and chemical targeting of the ATPase complex TIP48 and 49 impairs acute myeloid leukemia. Leukemia 37, 1812–1829 (2023). https://doi.org/10.1038/s41375-023-01971-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-01971-4