Abstract
LCK is a tyrosine kinase that is essential for initiating T-cell antigen receptor (TCR) signaling. A complete understanding of LCK function is constrained by a paucity of methods to quantitatively study its function within live cells. To address this limitation, we generated LCK*, in which a key active-site lysine is replaced by a photocaged equivalent, using genetic code expansion. This strategy enabled fine temporal and spatial control over kinase activity, thus allowing us to quantify phosphorylation kinetics in situ using biochemical and imaging approaches. We find that autophosphorylation of the LCK active-site loop is indispensable for its catalytic activity and that LCK can stimulate its own activation by adopting a more open conformation, which can be modulated by point mutations. We then show that CD4 and CD8, T-cell coreceptors, can enhance LCK activity, thereby helping to explain their effect in physiological TCR signaling. Our approach also provides general insights into SRC-family kinase dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Smith-Garvin, J.E., Koretzky, G.A. & Jordan, M.S. T cell activation. Annu. Rev. Immunol. 27, 591–619 (2009).
van der Merwe, P.A. & Dushek, O. Mechanisms for T cell receptor triggering. Nat. Rev. Immunol. 11, 47–55 (2011).
Ohashi, P.S. T-cell signalling and autoimmunity: molecular mechanisms of disease. Nat. Rev. Immunol. 2, 427–438 (2002).
Talab, F., Allen, J.C., Thompson, V., Lin, K. & Slupsky, J.R. LCK is an important mediator of B-cell receptor signaling in chronic lymphocytic leukemia cells. Mol. Cancer Res. 11, 541–554 (2013).
Tycko, B., Smith, S.D. & Sklar, J. Chromosomal translocations joining LCK and TCRB loci in human T cell leukemia. J. Exp. Med. 174, 867–873 (1991).
Xu, W., Doshi, A., Lei, M., Eck, M.J. & Harrison, S.C. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell 3, 629–638 (1999).
Boggon, T.J. & Eck, M.J. Structure and regulation of Src family kinases. Oncogene 23, 7918–7927 (2004).
Bergman, M. et al. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 11, 2919–2924 (1992).
Chow, L.M., Fournel, M., Davidson, D. & Veillette, A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature 365, 156–160 (1993).
Saunders, A.E. & Johnson, P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell. Signal. 22, 339–348 (2010).
Paster, W. et al. Genetically encoded Förster resonance energy transfer sensors for the conformation of the Src family kinase Lck. J. Immunol. 182, 2160–2167 (2009).
Nika, K. et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010).
Stirnweiss, A. et al. T cell activation results in conformational changes in the Src family kinase Lck to induce its activation. Sci. Signal. 6, ra13 (2013).
Hui, E. & Vale, R.D. In vitro membrane reconstitution of the T-cell receptor proximal signaling network. Nat. Struct. Mol. Biol. 21, 133–142 (2014).
Palacios, E.H. & Weiss, A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23, 7990–8000 (2004).
Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2, 309–322 (2002).
Veillette, A., Bookman, M.A., Horak, E.M. & Bolen, J.B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55, 301–308 (1988).
Rudd, C.E., Trevillyan, J.M., Dasgupta, J.D., Wong, L.L. & Schlossman, S.F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc. Natl. Acad. Sci. USA 85, 5190–5194 (1988).
Kim, P.W., Sun, Z.Y., Blacklow, S.C., Wagner, G. & Eck, M.J. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science 301, 1725–1728 (2003).
Dagliyan, O. et al. Engineering extrinsic disorder to control protein activity in living cells. Science 354, 1441–1444 (2016).
Karginov, A.V., Ding, F., Kota, P., Dokholyan, N.V. & Hahn, K.M. Engineered allosteric activation of kinases in living cells. Nat. Biotechnol. 28, 743–747 (2010).
Zhou, X.X., Fan, L.Z., Li, P., Shen, K. & Lin, M.Z. Optical control of cell signaling by single-chain photoswitchable kinases. Science 355, 836–842 (2017).
Davis, L. & Chin, J.W. Designer proteins: applications of genetic code expansion in cell biology. Nat. Rev. Mol. Cell Biol. 13, 168–182 (2012).
Chin, J.W. Expanding and reprogramming the genetic code of cells and animals. Annu. Rev. Biochem. 83, 379–408 (2014).
Gautier, A. et al. Genetically encoded photocontrol of protein localization in mammalian cells. J. Am. Chem. Soc. 132, 4086–4088 (2010).
James, J.R. & Vale, R.D. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature 487, 64–69 (2012).
Carrera, A.C., Alexandrov, K. & Roberts, T.M. The conserved lysine of the catalytic domain of protein kinases is actively involved in the phosphotransfer reaction and not required for anchoring ATP. Proc. Natl. Acad. Sci. USA 90, 442–446 (1993).
Gautier, A., Deiters, A. & Chin, J.W. Light-activated kinases enable temporal dissection of signaling networks in living cells. J. Am. Chem. Soc. 133, 2124–2127 (2011).
Schmied, W.H., Elsässer, S.J., Uttamapinant, C. & Chin, J.W. Efficient multisite unnatural amino acid incorporation in mammalian cells via optimized pyrrolysyl tRNA synthetase/tRNA expression and engineered eRF1. J. Am. Chem. Soc. 136, 15577–15583 (2014).
Williams, B.L. et al. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-γ1 and Ras activation. EMBO J. 18, 1832–1844 (1999).
Brdicka, T., Kadlecek, T.A., Roose, J.P., Pastuszak, A.W. & Weiss, A. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol. Cell. Biol. 25, 4924–4933 (2005).
Amrein, K.E. & Sefton, B.M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc. Natl. Acad. Sci. USA 85, 4247–4251 (1988).
Abraham, N. & Veillette, A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol. Cell. Biol. 10, 5197–5206 (1990).
Rossy, J., Owen, D.M., Williamson, D.J., Yang, Z. & Gaus, K. Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nat. Immunol. 14, 82–89 (2013).
Caron, L., Abraham, N., Pawson, T. & Veillette, A. Structural requirements for enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Mol. Cell. Biol. 12, 2720–2729 (1992).
Luo, K.X. & Sefton, B.M. Cross-linking of T-cell surface molecules CD4 and CD8 stimulates phosphorylation of the lck tyrosine protein kinase at the autophosphorylation site. Mol. Cell. Biol. 10, 5305–5313 (1990).
Chan, A.C. et al. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14, 2499–2508 (1995).
Watts, J.D. et al. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J. Biol. Chem. 269, 29520–29529 (1994).
Kong, G. et al. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol. Cell. Biol. 16, 5026–5035 (1996).
Denny, M.F., Patai, B. & Straus, D.B. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol. Cell. Biol. 20, 1426–1435 (2000).
Lovatt, M. et al. Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol. Cell. Biol. 26, 8655–8665 (2006).
Huse, M., Eck, M.J. & Harrison, S.C.A. A Zn2+ ion links the cytoplasmic tail of CD4 and the N-terminal region of Lck. J. Biol. Chem. 273, 18729–18733 (1998).
Vignali, D.A., Carson, R.T., Chang, B., Mittler, R.S. & Strominger, J.L. The two membrane proximal domains of CD4 interact with the T cell receptor. J. Exp. Med. 183, 2097–2107 (1996).
James, J.R., Oliveira, M.I., Carmo, A.M., Iaboni, A. & Davis, S.J. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat. Methods 3, 1001–1006 (2006).
Artyomov, M.N., Lis, M., Devadas, S., Davis, M.M. & Chakraborty, A.K. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc. Natl. Acad. Sci. USA 107, 16916–16921 (2010).
Brown, M.T. & Cooper, J.A. Regulation, substrates and functions of src. Biochim. Biophys. Acta 1287, 121–149 (1996).
Xu, B. & Miller, W.T. Src homology domains of v-Src stabilize an active conformation of the tyrosine kinase catalytic domain. Mol. Cell. Biochem. 158, 57–63 (1996).
Straus, D.B., Chan, A.C., Patai, B. & Weiss, A. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J. Biol. Chem. 271, 9976–9981 (1996).
Yamaguchi, H. & Hendrickson, W.A. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature 384, 484–489 (1996).
D'Oro, U., Sakaguchi, K., Appella, E. & Ashwell, J.D. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol. Cell. Biol. 16, 4996–5003 (1996).
Courtney, A.H. et al. A Phosphosite within the SH2 Domain of Lck regulates its activation by CD45. Mol. Cell 67, 498–511.e6 (2017).
Stepanek, O. et al. Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance. Cell 159, 333–345 (2014).
James, J.R. et al. The T cell receptor triggering apparatus is composed of monovalent or monomeric proteins. J. Biol. Chem. 286, 31993–32001 (2011).
Tan, C.W. et al. Wnt signalling pathway parameters for mammalian cells. PLoS One 7, e31882 (2012).
Rosenbluth, M.J., Lam, W.A. & Fletcher, D.A. Force microscopy of nonadherent cells: a comparison of leukemia cell deformability. Biophys. J. 90, 2994–3003 (2006).
Loiko, V.A. et al. Morphometric model of lymphocyte as applied to scanning flow cytometry. J. Quant. Spectrosc. Radiat. Transf. 102, 73–84 (2006).
Acknowledgements
This work was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number: 099966/Z/12/Z to J.R.J.) and by the Medical Research Council, UK (MC_U105181009 and MC_UP_A024_1008 to J.W.C.). We would like to thank the engineering workshop at the MRC-LMB for manufacturing the illumination device used in this study.
Author information
Authors and Affiliations
Contributions
A.L.-J., B.L.M. and J.R.J. designed and performed all of the experiments in the study. A.L.-J. and J.R.J. analyzed the data. M.M. synthesized the unnatural amino acid pc-Lys. J.W.C. provided scientific input and helped revise the manuscript, which was written by A.L.-J. and J.R.J. All authors contributed to the final manuscript. J.R.J. oversaw and supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
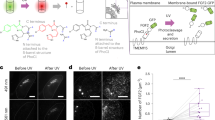
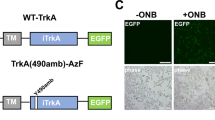
Supplementary Figure 1 Incorporation of photo-caged lysine (pc-Lys) at K273 of human LCK allows specific control of the enzyme activity.
(a) Modeling of the kinase domain of LCK with ATP (left) or pc-Lys at position 273 (right) in the native active site, based on a crystal structure of the kinase domain alone (1QPC). For the ATP-bound structure, the original AMP-PNP ligand has been modeled as ATP, with K273 also shown. For the pc-Lys model, the incorporated unnatural amino acid was built into the active site using PyMol. Rendering was performed in Chimera. (b) A representative western blot to show that the ZAP70 pY319 signal observed is specifically due to LCK* uncaging by UV illumination (350-400 nm). HEK-TCR cells were transfected with WT LCK* or kinase inactive LCK (K273R), ZAP70-mRuby2 and the components for pc-Lys incorporation, and illuminated with 350-400 nm or 500-550 nm light for 30 s as indicated in the left panel. Right panel shows the quantification of the western blot, data are normalized relative to final time point (15 min) of wildtype LCK* 350-400 nm and are presented as mean ± s.e.m. from independent experiments (n = 3). Uncropped blot images are shown in Supplementary Data Set 1.
Supplementary Figure 2 Expression of proteins in various cell lines.
(a) Histogram of flow cytometry data showing expression of cell surface TCR in cell lines indicated in the panel using a fluorescently conjugated anti-TCRβ antibody. (b) Histogram of flow cytometry data showing expression of endogenous CD45 phosphatase in HEK293T and Jurkat T cells using a fluorescently conjugated anti-CD45 antibody. (c) Normalized cytoplasmic densities of CSK, SHP-1 and SHP-2 expressed endogenously in cell lines indicated in the panels. Protein expression was detected by western blot using monoclonal antibodies specific to the proteins and data are normalized relative to the protein expression in CD4+ T cells. Quantification of the western blot is described in the Methods section. (d-f) Histograms of flow cytometry data showing cell surface expression of TCR (d), CD8 (e) and CD4 (f) in various HEK-derived cell lines using fluorescently conjugated antibodies against TCRβ, CD8β or CD4, respectively.
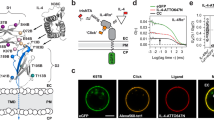
Supplementary Figure 3 Kinase activities of WT and mutant LCK* proteins on ZAP70 phosphorylation and recruitment to plasma membrane after uncaging.
(a) Plot of ZAP70 Y319 phosphorylation kinetics by wildtype LCK* from two identical sets of experiments with different numbers of time points collected, as indicated within the figure. (b) Plot of the kinetics of ZAP70 Y319 phosphorylation by wildtype or Y394F LCK* after uncaging, in the presence or absence of dominant negative version of CSK (K222R). Data in a and b are relative to final time point (15 min) of wildtype LCK* in each experiment and were fit using a 3-parameter logistic function, and are presented as mean ± s.e.m. from independent experiments (n = 4 in a [22 time points], n = 12 in a [6 time points] and n = 3 in b). (c,d) Quantification of the activities of wildtype and mutant LCK* kinases derived from the phosphorylation and recruitment kinetic curves. (c) Initial reaction rates (V0) of ZAP70 Y319 phosphorylation by LCK* mutants relative to WT LCK* after uncaging (related to Fig. 2b-d and 3a-d). (d) Time to achieve half-maximal recruitment of ZAP70 to plasma membrane by WT LCK* or indicated mutants after UV illumination at 5 mW/cm2 (related to Fig. 2f-h). For this measure of LCK* reaction rate, a lower value indicates more efficient kinase activity, which was not detectable (n.d.) for the Y394F mutant. Data are presented as mean ± s.e.m. from independent experiments (n = 4). (e) Identification of proline in the PxxP motif in the linker region of HCK (PDB ID: 1QCF) that binds intramolecularly to the SH3 domain. The amino acid numbering used here is based on human LCK sequence.
Supplementary Figure 4 Plot of the kinetics of ZAP70 Y319 phosphorylation by wildtype LCK*, FYN*, or SRC* after uncaging.
Data are relative to final time point (15 min) of LCK* and were fit using a 3-parameter logistic function. Data are presented as mean ± s.e.m. from independent experiments (n = 3).
Supplementary Figure 5 Time to achieve half-maximal recruitment of ZAP70 to phosphorylated ITAMs by wildtype LCK* in the presence of CD4 or CD8 co-receptors.
(a) Time to achieve half-maximal recruitment of ZAP70 to conjugate interface by WT LCK* in CD8+ HEK-TCRH and in HEK-TCRH cells after UV illumination at 5 mW/cm2 (related to Fig. 7b). For this measure of LCK* reaction rate, a lower value indicates more efficient kinase activity. (b,c) Time to achieve half-maximal recruitment of ZAP70 to plasma membrane by WT LCK* in the absence or presence of CD8 (b) or CD4 (c) in HEK-TCRH cells after UV illumination at 5 mW/cm2 for 2 s (related to Fig. 7e,f). (d,e) Time to achieve half-maximal recruitment of ZAP70 to plasma membrane by WT LCK* in CD8+ and CD86ExCD8Int+ (d), or CD4+ and CD86ExCD4Int+ (e) HEK‑TCRH cells relative to that in HEK‑TCRH cells after UV illumination at 5 mW/cm2 for 2 s (related to Fig. 7g,h). (f,g) Microscopy image quantification showing ZAP70 recruitment to plasma membrane after LCK* uncaging in the presence or absence of CD8 (f) or CD4 (g) in HEK-TCRH cells after UV illumination at 500 mW/cm2 for 2 s. Data are normalized to maximum asymptote values for each dataset. Lines show data smoothed used a moving-average filter, data points represent mean and filled areas represent s.e.m. from independent experiments (n = 3), where 4-8 cells were used in each independent experiment. (h,i) Time to achieve half-maximal recruitment of ZAP70 to plasma membrane by WT LCK* in the absence or presence of CD8 (h) or CD4 (i) in HEK-TCRH cells after UV illumination at 500 mW/cm2 for 2 s (related to Supplementary Fig. 5f,g). Data in a-e,h,i are presented as mean ± s.e.m. from independent experiments (n=4 in a-c; 3 in d,e,h,i).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 706 kb)
Supplementary Data Set 1
Uncropped grayscale images of Western blots shown in the main figures. The Western blots in the main figures are presented as merged images of the 700 nm channel (red) and 800 nm channel (green). (PDF 852 kb)
Supplementary Data Set 2
Source data for all figures. (XLSX 378 kb)
Differential rate of ZAP70 membrane translocation after uncaging of LCK* mutants.
A combined video showing ZAP70-mRuby2 translocation from cytosol to phosphorylated ITAMs at the plasma membrane of HEKTCRH cells by wildtype or mutant LCK* as indicated in the video. LCK* photo-uncaging by illumination for 2 s at 5 mW/cm2 (t = 0 s) is marked by the white box. Video is shown at 10× real-time (7.1 fps) and scale bar represents 5 μm. Video is related to Fig. 2e. (MP4 1391 kb)
ZAP70 recruitment to a spatio-temporally defined sub-cellular region of a cell conjugate.
Representative time lapse video of ZAP70-mRuby2 translocation from cytosol to phosphorylated ITAMs at a specific cell conjugate region between HEK-TCRH and Raji B cell expressing pMHC-BFP after uncaging of wildtype LCK*. A diffraction-limited spot in the upper conjugate shown was exposed to focused 405 nm laser pulses (10×100 μs) to uncage LCK* in that region only, as marked by the appearance of a white arrow in ZAP70 panel at -1 s. Colored labels denote protein representation in the overlay. Video is shown at 10× real-time (10 fps) and scale bar represents 5 μm. Video is related to Fig. 6. (MP4 5125 kb)
ZAP70 recruitment to cell conjugate region after wildtype LCK* uncaging.
Representative time-lapse video of ZAP70-mRuby2 translocation from cytosol to phosphorylated ITAMs at the cell conjugate region between HEK-TCRH and Raji B cell expressing pMHC-BFP after uncaging of wildtype LCK*. LCK* photo-uncaging by illumination for 2 s at 5 mW/cm2 (t = 0 s) is marked by the white box. Colored labels denote protein representation in the overlay. Video is shown at 10× real-time (7.1 fps) and scale bar represents 5 μm. Video is related to Fig. 7a. (MP4 1871 kb)
Rights and permissions
About this article
Cite this article
Liaunardy-Jopeace, A., Murton, B., Mahesh, M. et al. Encoding optical control in LCK kinase to quantitatively investigate its activity in live cells. Nat Struct Mol Biol 24, 1155–1163 (2017). https://doi.org/10.1038/nsmb.3492
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3492
This article is cited by
-
DeKinomics pulse-chases kinase functions in living cells
Nature Chemical Biology (2024)
-
LCK facilitates DNA damage repair by stabilizing RAD51 and BRCA1 in the nucleus of chemoresistant ovarian cancer
Journal of Ovarian Research (2023)
-
An immunomodulating peptide with potential to suppress tumour growth and autoimmunity
Scientific Reports (2023)
-
Unique roles of co-receptor-bound LCK in helper and cytotoxic T cells
Nature Immunology (2023)
-
Bound to be perfect: Lck and T cell co-receptors
Nature Immunology (2023)