Abstract
The DNA-damage response (DDR) ensures genome stability and proper inheritance of genetic information, both of which are essential to survival. It is presently unclear to what extent other signaling pathways modulate DDR function. Here we show that Notch receptor binds and inactivates ATM kinase and that this mechanism is evolutionarily conserved in Caenorhabditis elegans, Xenopus laevis and humans. In C. elegans, the Notch pathway impairs DDR signaling in gonad germ cells. In mammalian cells, activation of human Notch1 leads to reduced ATM signaling in a manner independent of Notch1 transcriptional activity. Notch1 binds directly to the regulatory FATC domain of ATM and inhibits ATM kinase activity. Notch1 and ATM activation are inversely correlated in human breast cancers, and inactivation of ATM by Notch1 contributes to the survival of Notch1-driven leukemia cells upon DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
d'Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522 (2008).
Jackson, S.P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Bailly, A. & Gartner, A. Germ cell apoptosis and DNA damage responses. Adv. Exp. Med. Biol. 757, 249–276 (2013).
Stergiou, L. & Hengartner, M.O. Death and more: DNA damage response pathways in the nematode C. elegans. Cell Death Differ. 11, 21–28 (2004).
Ntziachristos, P., Lim, J.S., Sage, J. & Aifantis, I. From fly wings to targeted cancer therapies: a centennial for Notch signaling. Cancer Cell 25, 318–334 (2014).
Andersson, E.R., Sandberg, R. & Lendahl, U. Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612 (2011).
Andersen, P., Uosaki, H., Shenje, L.T. & Kwon, C. Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol. 22, 257–265 (2012).
Koch, U. & Radtke, F. Notch and cancer: a double-edged sword. Cell. Mol. Life Sci. 64, 2746–2762 (2007).
Ellisen, L.W. et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66, 649–661 (1991).
Vermezovic, J., Stergiou, L., Hengartner, M.O. & d'Adda di Fagagna, F. Differential regulation of DNA damage response activation between somatic and germline cells in Caenorhabditis elegans. Cell Death Differ. 19, 1847–1855 (2012).
Pepper, A.S., Lo, T.W., Killian, D.J., Hall, D.H. & Hubbard, E.J. The establishment of Caenorhabditis elegans germline pattern is controlled by overlapping proximal and distal somatic gonad signals. Dev. Biol. 259, 336–350 (2003).
Kodoyianni, V., Maine, E.M. & Kimble, J. Molecular basis of loss-of-function mutations in the glp-1 gene of Caenorhabditis elegans. Mol. Biol. Cell 3, 1199–1213 (1992).
Rustighi, A. et al. The prolyl-isomerase Pin1 is a Notch1 target that enhances Notch1 activation in cancer. Nat. Cell Biol. 11, 133–142 (2009).
Rand, M.D. et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol. Cell. Biol. 20, 1825–1835 (2000).
Schmitt, T.M. & Zuniga-Pflucker, J.C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17, 749–756 (2002).
Perumalsamy, L.R., Nagala, M., Banerjee, P. & Sarin, A. A hierarchical cascade activated by non-canonical Notch signaling and the mTOR-Rictor complex regulates neglect-induced death in mammalian cells. Cell Death Differ. 16, 879–889 (2009).
Borggrefe, T. & Oswald, F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell. Mol. Life Sci. 66, 1631–1646 (2009).
Westhoff, B. et al. Alterations of the Notch pathway in lung cancer. Proc. Natl. Acad. Sci. USA 106, 22293–22298 (2009).
Palomero, T. et al. CUTLL1, a novel human T-cell lymphoma cell line with t(7;9) rearrangement, aberrant NOTCH1 activation and high sensitivity to gamma-secretase inhibitors. Leukemia 20, 1279–1287 (2006).
Weng, A.P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004).
Khanna, K.K. et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat. Genet. 20, 398–400 (1998).
Jiang, X., Sun, Y., Chen, S., Roy, K. & Price, B.D. The FATC domains of PIKK proteins are functionally equivalent and participate in the Tip60-dependent activation of DNA-PKcs and ATM. J. Biol. Chem. 281, 15741–15746 (2006).
Garner, E. & Costanzo, V. Studying the DNA damage response using in vitro model systems. DNA Repair (Amst.) 8, 1025–1037 (2009).
Smith, G.C. et al. Purification and DNA binding properties of the ataxia-telangiectasia gene product ATM. Proc. Natl. Acad. Sci. USA 96, 11134–11139 (1999).
Grabher, C., von Boehmer, H. & Look, A.T. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat. Rev. Cancer 6, 347–359 (2006).
Stylianou, S., Clarke, R.B. & Brennan, K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 66, 1517–1525 (2006).
Farnie, G. et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J. Natl. Cancer Inst. 99, 616–627 (2007).
Bisso, A. et al. Oncogenic miR-181a/b affect the DNA damage response in aggressive breast cancer. Cell Cycle 12, 1679–1687 (2013).
Rustighi, A. et al. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO Mol. Med. 6, 99–119 (2014).
Elkon, R. et al. Dissection of a DNA-damage-induced transcriptional network using a combination of microarrays, RNA interference and computational promoter analysis. Genome Biol. 6, R43 (2005).
Rashi-Elkeles, S. et al. Parallel induction of ATM-dependent pro- and antiapoptotic signals in response to ionizing radiation in murine lymphoid tissue. Oncogene 25, 1584–1592 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Kimble, J. & Crittenden, S.L. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 23, 405–433 (2007).
Fassl, A. et al. Notch1 signaling promotes survival of glioblastoma cells via EGFR-mediated induction of anti-apoptotic Mcl-1. Oncogene 31, 4698–4708 (2012).
Wang, J. et al. Notch promotes radioresistance of glioma stem cells. Stem Cells 28, 17–28 (2010).
Sade, H., Krishna, S. & Sarin, A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J. Biol. Chem. 279, 2937–2944 (2004).
Beverly, L.J., Felsher, D.W. & Capobianco, A.J. Suppression of p53 by Notch in lymphomagenesis: implications for initiation and regression. Cancer Res. 65, 7159–7168 (2005).
Gartner, A., Milstein, S., Ahmed, S., Hodgkin, J. & Hengartner, M.O. A conserved checkpoint pathway mediates DNA damage–induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 5, 435–443 (2000).
Schumacher, B., Hofmann, K., Boulton, S. & Gartner, A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722–1727 (2001).
Wang, J. et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell 148, 1001–1014 (2012).
Schneider, L. et al. DNA damage in mammalian neural stem cells leads to astrocytic differentiation mediated by BMP2 signaling through JAK-STAT. Stem Cell Reports 1, 123–138 (2013).
Kim, S.B. et al. Activated Notch1 interacts with p53 to inhibit its phosphorylation and transactivation. Cell Death Differ. 14, 982–991 (2007).
Tavtigian, S.V. et al. Rare, evolutionarily unlikely missense substitutions in ATM confer increased risk of breast cancer. Am. J. Hum. Genet. 85, 427–446 (2009).
Groth, C. & Fortini, M.E. Therapeutic approaches to modulating Notch signaling: current challenges and future prospects. Semin. Cell Dev. Biol. 23, 465–472 (2012).
Politi, K., Feirt, N. & Kitajewski, J. Notch in mammary gland development and breast cancer. Semin. Cancer Biol. 14, 341–347 (2004).
Campisi, J. & d′Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Halazonetis, T.D., Gorgoulis, V.G. & Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 319, 1352–1355 (2008).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Francis, R., Barton, M.K., Kimble, J. & Schedl, T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579–606 (1995).
Lee, M.H. & Schedl, T. Identification of in vivo mRNA targets of GLD-1, a maxi-KH motif containing protein required for C. elegans germ cell development. Genes Dev. 15, 2408–2420 (2001).
Ciofani, M. et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J. Immunol. 172, 5230–5239 (2004).
Carpenter, A.E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Fitzgerald, D.J. et al. Protein complex expression by using multigene baculoviral vectors. Nat. Methods 3, 1021–1032 (2006).
Hashimoto, Y. & Costanzo, V. Studying DNA replication fork stability in Xenopus egg extract. Methods Mol. Biol. 745, 437–445 (2011).
Francia, S. et al. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 488, 231–235 (2012).
Robertson, K., Hensey, C. & Gautier, J. Isolation and characterization of Xenopus ATM (X-ATM): expression, localization, and complex formation during oogenesis and early development. Oncogene 18, 7070–7079 (1999).
Irizarry, R.A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Tusher, V.G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98, 5116–5121 (2001).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Fallarino, F. et al. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat. Med. 16, 897–902 (2010).
Johnson, W.E., Li, C. & Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2007).
Rustighi, A. et al. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO Mol. Med. 6, 99–119 (2014).
Adorno, M. et al. A mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell 137, 87–98 (2009).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
We thank T. Schedl (Washington University School of Medicine), M. Donzelli (Istituto Europeo di Oncologia), P.P. Di Fiore (Istituto Europeo di Oncologia), N. Offenhaeuser (Istituto Fondazione Italiana per la Ricerca sul Cancro di Oncologia Molecolare), S. Pece (Istituto Europeo di Oncologia), J.C. Zuniga-Pflucker (Sunnybrook and Women's College Health Sciences Center), P.G. Pelicci (Istituto Europeo di Oncologia), A. Sarin (National Centre for Biological Sciences), A. Behrens (London Research Institute), T. Halazonetis (University of Geneva), A. Ferrando (Columbia University) and W.S. Pear (Abramson Family Cancer Research Institute) for reagents; T. Vaccari, F. Kobia, A. Petrovic and A. Musacchio for advice; G. Bottai and G. Bianchini for technical assistance with IHC experiments; and S. Pasqualato and S. Monzani for help with purification of human recombinant Notch1. L.S. is supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC; grant 6251). S.B. and M.F. are supported by funds from the AIRC Special Program Molecular Clinical Oncology '5 per mille' and from Fondo per gli Investimenti della Ricerca di Base (FIRB; Accordi di Programma 2011 RBAP11T3WB). G.D.S. is supported by grants from the AIRC Special Program Molecular Clinical Oncology '5 per mille' and from the Italian University and Research Ministerium (Cofin FIRB). F.d'A.d.F. is supported by the Fondazione Italiana per la Ricerca sul Cancro, AIRC (application 12971), Human Frontier Science Program (contract RGP 0014/2012), Cariplo Foundation (grant 2010.0818), Marie Curie Initial Training Networks (FP7 PEOPLE 2012 ITN (CodAge)), Fondazione Telethon (GGP12059), Association for International Cancer Research, Progetti di Ricerca di Interesse Nazionale (PRIN) 2010–2011, the Italian Ministry of Education Universities and Research EPIGEN Project, and an European Research Council advanced grant (322726).
Author information
Authors and Affiliations
Contributions
F.d'A.d.F. and J.V. designed the study. J.V., M.A., L.S., M.F. and A.R. performed the experiments. J.V., M.A., L.S., M.F., S.B., A.R., G.D.S., V.C. and F.d'A.d.F. analyzed the data. L.M. performed baculovirus infections of insect cells, and C.L. helped with coculture of OP9-DL1 cells. F.d'A.d.F. and J.V. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Notch shows inverse correlation with ATM activity in C. elegans and negatively regulates ATM activity in human cells.
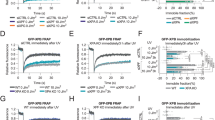
(a) Scheme of C. elegans germline. Two large pools of cells are outlined: the proliferating germ stem cells and cells undergoing meiotic differentiation. Distal tip cell (DTC) that caps the germline maintains stemness through Notch signaling. (b) RNA in situ hybridization of dissected gonads of wild-type worms with antisense or sense probes for atm-1 and gld-1 transcripts. gld-1 used here as a control, is predominantly expressed in the meiotic compartment. (c) Immunostaining for pS/TQ epitopes of temperature-sensitive glp-1(ar202) mutant grown at 25°C and then kept at 15°C for 5h or 20h prior to exposure to IR. (d) Bar plot presents quantification of the percentage of cells positive for the indicated DDR marker presented in Fig. 1c. We considered only foci like stainings for quantifications. *P value ≤ 0.001. (e) Fields of immunostained Hela cells expressing N1ΔE for indicated DDR markers and shown in Fig. 1c. (f) Immunostaining for Notch1 and pCHK2T68 of Hela cells expressing N1ΔE, after IR (2 Gy). (g) Bar plot presents quantification of the percentage of cells positive for pCHK2T68. *P value ≤ 0.001. (h) Immunostaining for Notch1 and pKAP1S824 of Hela cells expressing N1ΔE, after IR (2 Gy). (i) Bar plot presents quantification of the percentage of cells positive for pKAP1S824. *P value ≤ 0.001. (j) Immunostaining for Notch1 and pSMC1S966 of Hela cells expressing N1ΔE after IR (2 Gy). (k) Bar plot presents quantification of the percentage of cells positive for pSMC1S966. *P value ≤ 0.001. (l) Immunostaining for Notch1 and 53BP1 of Hela cells expressing N1ΔE, after exposure to IR (2 Gy). Cells were collected at different time points (1, 4 and 24 hours) after IR and immunostained. (m-n) Bar plots indicate percentages of cells positive for 53BP1 and pATM nuclear foci, at different time points after exposure to IR. *P value ≤ 0.001. (o) Bar plot shows mean signal intensity of DDR foci per nucleus upon Notch induction. Endogenous Notch1 activation was induced by EGTA treatment in MCF10a cells. Cells were then exposed to IR (0.5 Gy) and immunostained for indicated DDR markers. All values were normalized to those of uninduced cells. *P value ≤ 0.05. (p) Immunostaining for N1IC of MCF10a cells cocultured either with OP9 cells or OP9 cells expressing DL1-GFP ligand. (q) Bar plot shows efficacy of GSI treatment, estimated by qRT-PCR for Notch1 transcriptional target Hes1. The values were normalized to the levels of Hes1 expression in empty vector infected cells. (r) Immunoblot for indicated DDR markers on lysates of HeLa cells infected with either empty vector or one expressing N1IC. Cells were sorted for homogeneous GFP signal and exposed to IR (5 Gy). (s) Bar plot presents quantification of the signal intensities shown on the immunoblot in (r). Signal intensities of DDR markers were normalized to that of respective total proteins and to the values of empty vector infected cells. *P value ≤ 0.05. (t) Immunoblot for marker of apoptosis cleaved caspase-3 on lysates of Hela cells expressing N1IC. Cells were exposed to IR (20 Gy) and collected 24 hours after for the analysis. (u) Immunoblot for pATM on lysates of MCF10a cells infected with either empty vector or one expressing N1IC. Cells were sorted for homogeneous GFP signal and exposed to IR (5 Gy). (v) Bar plot presents quantification of the signal intensities of DDR markers shown on the immunoblot in (u). Intensity of pATM signal was normalized to that of total ATM. Values were further normalized to that of empty vector infected cells. *P value ≤ 0.05. Throughout figure, error bars represent s.e.m. (n = 3 independent experiments), and all P values were calculated by two-tailed Student’s t test.
Supplementary Figure 2 Inactivation of DDR requires nuclear localization of Notch1, but it is not dependent on Notch1 transcriptional activity.
(a), (c) and (e) Immunostainings for indicated DDR markers on Hela cells transfected with membrane-bound CD8-N1IC-GFP or cytosolic N1IC-NES-GFP or nuclear N1IC-NLS-GFP and exposed to IR (2 Gy). (b), (d) and (f) Bar plots present quantifications of the percentages of cells positive for indicated DDR marker; Error bars, s.e.m. n = 3 independent experiments; (ns P value > 0.05; *P value ≤ 0.05; **P value ≤ 0.001) (g) Bar plot presents quantification of the signal intensities shown on immunoblot in Fig. 2c. Intensity of pATM signal was normalized to that of total ATM and to the values of mock vector transfected cells. (h) Bar plot presents quantification of the levels of expression of Notch1 transcription target Hes1, as measured by qRT-PCR, in Hela cells expressing Notch1WT or transcriptionally inactivated Notch1ΔTAD. The values were normalized to the levels of Hes1 expression in Notch1WT cells. (i) Bar plot presents quantification of the signal intensities shown on the immunoblot in Fig. 2g. Intensity of total ATM signal was normalized to that of the vinculin and to the values of cells expressing GFP only. All P values shown in figure 2 are calculated by two-tailed Student’s t test.
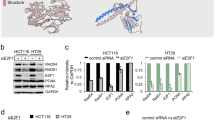
Supplementary Figure 3 Notch1 and ATM form a protein complex.
(a) Immunoblot analysis of an IP with an anti-Flag antibody from the lysates of 293T cells expressing ATM-Flag and N1ΔE-Myc; input 5% of the total lysate. (b) Immunoblot analysis of an IP with an anti-Flag antibody from the lysates of 293T cells expressing N1ΔE-Flag. Lysates were subjected to either ethidium bromide or DNase treatment; input: 5% of the total lysate. (c) and (d) Immunoblot analysis of IPs with antibodies against endogenous ATM or anti-Flag (for Notch1) from the lysates of 293T cells expressing either N1ΔE-Flag or control-Flag tagged protein (Meis1-Flag). Cells were exposed to IR prior to collection of lysates. (e) Immunoblot analysis of an IP with an antibody against ATM from the lysates of 293T cells expressing N1ΔE-Flag. Levels of NBS1 remain unaffected in the presence of Notch1; input: 5% of the total lysate. (f) in situ proximity ligation assay (Duolink) performed on HeLa cells expressing empty vector or N1ΔE, shows detectable bright fluorescent signal where Notch1 and ATM are in close proximity.
Supplementary Figure 4 Notch1 binds directly to ATM and inactivates it.
(a) Immunoblot analysis of an IP with an anti-Flag antibody from the lysates of 293T cells expressing either mock-Flag protein (Meis1-Flag), N1ΔE-Flag or N1ΔEΔANK-Flag; input: 5% of the total lysate. (b) Bar plot presents quantification of the percentages of cells positive for pATM foci in Hela cells expressing either N1ΔE or N1ΔEΔANK, and after exposure to IR (2 Gy). Error bars, s.e.m.; n = 3 independent experiments (*P value ≤ 0.01). (c) Silver staining of SDS-PAGE protein gel shows a single band for ATM, immunoprecipitated from lysates of HeLa cells. (d) Bar plot presents quantification of the signal intensities shown on the immunoblot in Fig. 4d. Intensity of p53S15 signal was normalized to the intensity of total ATM signal, GST alone and finally to the values of the reaction without N1IC. Error bars, s.e.m.; n = 3 independent experiments (ns P value > 0.05; *P value ≤ 0.05). All P values shown in figure 2 are calculated by two-tailed Student’s t test.
Supplementary Figure 5 Inhibition of Notch1, in the presence of IR, leads to increased apoptosis and reduced survival of TALL-1 cells.
(a) Bar plot shows efficacy of GSI treatment, estimated by qRT-PCR for Notch1 transcriptional target Hes1. The values were normalized to the levels of Hes1 expression in DMSO-treated cells. (b) Immunoblot confirms efficient treatment of TALL-1 cells with ATM inhibitor (ATMi). TALL-1 cells were treated with ATMi for 3h prior to IR (3 Gy). (c) Bar plot shows efficacy of GSI treatment, estimated by qRT-PCR for Notch1 transcriptional target Hes1. The values were normalized to the levels of Hes1 expression in DMSO-treated cells.
Supplementary Figure 6 Notch1 and ATM are inversely correlated in human breast cancer samples.
(a) Immunohistochemistry (IHC) images show examples of low-grade breast cancers that express low levels of nuclear Nocth1 and high levels of pATM. Conversely, high-grade breast cancers show high levels of detectable nuclear Notch1 and low levels of pATM. (b) IHC of parallel sections of one breast cancer sample shows nuclear activated Notch1 (score 3), full expression of nuclear ATM (score 3) and significant reduction of nuclear pATM (score 1). (c) Scatter plot shows inverse correlation between Notch1 and pATM, when samples that do not express total ATM (7%) are removed from the analysis; P < 0.009343 (d) Box plots show levels of Notch1 and pATM stainings for the indicated breast cancer molecular subtypes: HER2 positive, Luminal A, Luminal B, Triple Negative (TN). Inverse correlation between Notch1 and pATM is maintained also among different molecular subtypes of breast cancer. Notch1 plot: P < 0.004064; pATM plot: P < 0.0536; Kruskal-Wallis chi-squared test was used for this analysis. (e) Kaplan-Meier graph representing the probability of overall survival in breast cancer patients from the meta-dataset stratified according to NDT and ATM-activity signatures. The log-rank test P value reflects the significance of the association between NDT signature Low/ATM-activity signature High and longer survival.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 4756 kb)
Supplementary Table 1
Breast cancer reorganized cohorts comprised in the metadataset analyzed in this study (PDF 117 kb)
Supplementary Table 2
ATM-activity gene signature (PDF 101 kb)
Supplementary Data Set 1
Original immunoblot images (PDF 2500 kb)
Rights and permissions
About this article
Cite this article
Vermezovic, J., Adamowicz, M., Santarpia, L. et al. Notch is a direct negative regulator of the DNA-damage response. Nat Struct Mol Biol 22, 417–424 (2015). https://doi.org/10.1038/nsmb.3013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3013
This article is cited by
-
The role of Hedgehog and Notch signaling pathway in cancer
Molecular Biomedicine (2022)
-
Spatial regulation and generation of diversity in signaling pathways
Journal of Biosciences (2021)
-
Effects of β-HPV on DNA damage response pathways to drive carcinogenesis: a review
Virus Genes (2021)
-
Multi-platform profiling characterizes molecular subgroups and resistance networks in chronic lymphocytic leukemia
Nature Communications (2021)
-
NOTCH1 gene amplification promotes expansion of Cancer Associated Fibroblast populations in human skin
Nature Communications (2020)