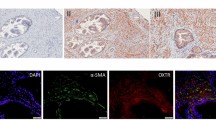
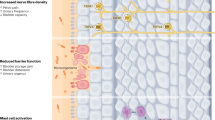
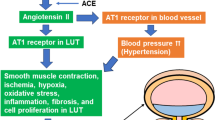
Key Points
-
The nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic GMP (cGMP) signalling pathway is present in the smooth muscle of the lower urinary tract (bladder and urethra), prostate, and corpus cavernosum
-
Impairment of NO–sGC–cGMP signalling in bladder, urethra, prostate, and corpus cavernosum smooth muscles can result in urogenital diseases such as overactive bladder, prostate hypercontractility, and erectile dysfunction
-
Stimulators (BAY 41–2272, BAY 41–8543, and BAY 60–4552) and activators (BAY 58–2667 and BAY 60–2770) of sGC can enhance cGMP levels by directly acting on sGC independently of NO
-
Both sGC stimulators and activators have a potent relaxation effect on bladder, ureter, urethra, prostate, and corpus cavernosum smooth muscle in nonpathological and pathological conditions
-
sGC activators are highly effective at restoring sGC expression and activity in disorders associated with increased oxidative stress involving urogenital smooth muscle. sGC activators are more efficacious than sGC stimulators
-
sGC stimulators and activators are promising molecules that enable urogenital smooth muscle relaxation in pathological conditions. However, clinical studies are required to corroborate the efficacy of these drugs
Abstract
Lower urinary tract symptoms (LUTS), comprising storage (such as urinary incontinence and urinary frequency), voiding, and postmicturition symptoms, are highly prevalent conditions that affect millions of people worldwide. LUTS have a profound effect on quality of life and are a considerable cost to health care systems. In men specifically, BPH commonly leads to LUTS. Clinical studies also show an association of LUTS with erectile dysfunction (ED). Nitric oxide (NO) has long been recognized as an important nonadrenergic, noncholinergic (NANC) transmitter in bladder, urethra, prostate, and corpus cavernosum smooth muscle. Data from clinical and basic research show that oxidation and degradation of soluble guanylate cyclase (sGC; also known as GCS) and reduced cyclic GMP (cGMP) levels are involved in the physiopathology of genitourinary diseases. The NO–sGC–cGMP signalling pathway has a role in disease pathophysiology of the bladder, urethra, prostate, and corpus cavernosum in animal models and humans. Advances in targeting sGC directly to enhance cGMP production independently of endogenous NO have been made using NO-independent stimulators and activators of sGC. These molecules are potential therapeutics in the treatment of LUTS and ED.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Abrams, P. et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 21, 167–178 (2002).
Andersson, K. E. & Wein, A. J. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol. Rev. 56, 581–631 (2004).
Murad, F., Mittal, C. K., Arnold, W. P., Katsuki, S. & Kimura, H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv. Cycl. Nucleotide Res. 9, 145–158 (1978).
Francis, S. H., Busch, J. L. & Corbin, J. D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 (2010).
Gold, M. E., Wood, K. S., Buga, G. M., Byrns, R. E. & Ignarro, L. J. L-Arginine causes whereas L-argininosuccinic acid inhibits endothelium-dependent vascular smooth muscle relaxation. Biochem. Biophys. Res. Commun. 161, 536–543 (1989).
Palmer, R. M., Ferrige, A. G. & Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 (1987).
Forstermann, U. et al. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem. Pharmacol. 42, 1849–1857 (1991).
Griscavage, J. M., Rogers, N. E., Sherman, M. P. & Ignarro, L. J. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J. Immunol. 151, 6329–6337 (1993).
Derbyshire, E. R. & Marletta, M. A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 81, 533–559 (2012).
Rapoport, R. M., Draznin, M. B. & Murad, F. Sodium nitroprusside-induced protein phosphorylation in intact rat aorta is mimicked by 8-bromo cyclic GMP. Proc. Natl Acad. Sci. USA 79, 6470–6474 (1982).
Persson, K., Alm, P., Johansson, K., Larsson, B. & Andersson, K. E. Nitric oxide synthase in pig lower urinary tract: immunohistochemistry, NADPH diaphorase histochemistry and functional effects. Br. J. Pharmacol. 110, 521–530 (1993).
Kots, A. Y., Bian, K. & Murad, F. Nitric oxide and cyclic GMP signaling pathway as a focus for drug development. Curr. Med. Chem. 18, 3299–3305 (2011).
Mendes-Silverio, C. B. et al. Activation of haem-oxidized soluble guanylyl cyclase with BAY 60–2770 in human platelets lead to overstimulation of the cyclic GMP signaling pathway. PLoS ONE 7, e47223 (2012).
Conran, N. et al. Nitric oxide regulates human eosinophil adhesion mechanisms in vitro by changing integrin expression and activity on the eosinophil cell surface. Br. J. Pharmacol. 134, 632–638 (2001).
Jenei, V. et al. Nitric oxide produced in response to engagement of β2 integrins on human neutrophils activates the monomeric GTPases Rap1 and Rap2 and promotes adhesion. J. Biol. Chem. 281, 35008–35020 (2006).
Thomazzi, S. M., Moreira, J., Marcondes, S., De Nucci, G. & Antunes, E. Role of cyclic GMP on inhibition by nitric oxide donors of human eosinophil chemotaxis in vitro. Br. J. Pharmacol. 141, 653–660 (2004).
Zhu, H. et al. Restoring soluble guanylyl cyclase expression and function blocks the aggressive course of glioma. Mol. Pharmacol. 80, 1076–1084 (2011).
Borst, P., de Wolf, C. & van de Wetering, K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 453, 661–673 (2007).
Kanamasa, K. et al. Eccentric dosing of nitrates does not increase cardiac events in patients with healed myocardial infarction. Hypertens. Res. 27, 563–572 (2004).
Bian, K., Ke, Y., Kamisaki, Y. & Murad, F. Proteomic modification by nitric oxide. J. Pharmacol. Sci. 101, 271–279 (2006).
Evgenov, O. V. et al. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat. Rev. Drug Discov. 5, 755–768 (2006).
Schroder, A., Hedlund, P. & Andersson, K. E. Carbon monoxide relaxes the female pig urethra as effectively as nitric oxide in the presence of YC-1. J. Urol. 167, 1892–1896 (2002).
Mizusawa, H., Hedlund, P., Brioni, J. D., Sullivan, J. P. & Andersson, K. E. Nitric oxide independent activation of guanylate cyclase by YC-1 causes erectile responses in the rat. J. Urol. 167, 2276–2281 (2002).
Bau, F. R. et al. Evaluation of the relaxant effect of the nitric oxide-independent soluble guanylyl cyclase stimulator BAY 41–2272 in isolated detrusor smooth muscle. Eur. J. Pharmacol. 637, 171–177 (2010).
Mónica, F. Z. et al. Long-term nitric oxide deficiency causes muscarinic supersensitivity and reduces β3-adrenoceptor-mediated relaxation, causing rat detrusor overactivity. Br. J. Pharmacol. 153, 1659–1668 (2008).
Fullhase, C. et al. Reduction of obstruction related bladder overactivity by the guanylyl cyclase modulators BAY 41–2272 and BAY 60–2770 alone or in combination with a phosphodiesterase type 5 inhibitor. Neurourol. Urodyn. 34, 787–793 (2015).
Toque, H. A., Antunes, E., Teixeira, C. E. & De Nucci, G. Increased cyclic guanosine monophosphate synthesis and calcium entry blockade account for the relaxant activity of the nitric oxide-independent soluble guanylyl cyclase stimulator BAY 41–2272 in the rabbit penile urethra. Urology 72, 711–715 (2008).
Alexandre, E. C. et al. Soluble guanylyl cyclase (sGC) degradation and impairment of nitric oxide-mediated responses in urethra from obese mice: reversal by the sGC activator BAY 60–2770. J. Pharmacol. Exp. Ther. 349, 2–9 (2014).
Calmasini, F. B. et al. Soluble guanylate cyclase modulators, BAY 41–2272 and BAY 60–2770, inhibit human and rabbit prostate contractility. Urology 94, 312.e9–312.e15 (2016).
Miyaoka, R. et al. BAY 41–2272, a soluble guanylate cyclase stimulator, relaxes isolated human ureter in a standardized in vitro model. Urology 83, 256.e1–256.e7 (2014).
Oudot, A. et al. Combination of BAY 60–4552 and vardenafil exerts proerectile facilitator effects in rats with cavernous nerve injury: a proof of concept study for the treatment of phosphodiesterase type 5 inhibitor failure. Eur. Urol. 60, 1020–1026 (2011).
Lasker, G. F. et al. Analysis of erectile responses to BAY 41–8543 and muscarinic receptor stimulation in the rat. J. Sex. Med. 10, 704–718 (2013).
Silva, F. H. et al. Oxidative stress associated with middle aging leads to sympathetic hyperactivity and downregulation of soluble guanylyl cyclase in corpus cavernosum. Am. J. Physiol. Heart Circ. Physiol. 307, H1393–1400 (2014).
Decaluwe, K. et al. Erectile dysfunction in heme-deficient nitric oxide-unresponsive soluble guanylate cyclase knock-in mice. J. Sex. Med. 14, 196–204 (2017).
Estancial, C. S., Rodrigues, R. L., De Nucci, G., Antunes, E. & Mónica, F. Z. Pharmacological characterisation of the relaxation induced by the soluble guanylate cyclase activator, BAY 60–2770 in rabbit corpus cavernosum. BJU Int. 116, 657–664 (2015).
Albersen, M., Linsen, L., Tinel, H., Sandner, P. & Van Renterghem, K. Synergistic effects of BAY 60–4552 and vardenafil on relaxation of corpus cavernosum tissue of patients with erectile dysfunction and clinical phosphodiesterase type 5 inhibitor failure. J. Sex. Med. 10, 1268–1277 (2013).
Wedel, B. et al. Mutation of His-105 in the β1 subunit yields a nitric oxide-insensitive form of soluble guanylyl cyclase. Proc. Natl Acad. Sci. USA 91, 2592–2596 (1994).
Stasch, J. P. et al. NO-independent regulatory site on soluble guanylate cyclase. Nature 410, 212–215 (2001).
Stasch, J. P. et al. Pharmacological actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: in vitro studies. Br. J. Pharmacol. 135, 333–343 (2002).
Stasch, J. P. et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Invest. 116, 2552–2561 (2006).
Schmidt, H. H. H. W., Schmidt, P. M. & Stasch, J. P. in Handbook of Experimental Pharmacology Vol. 191 (eds Schmidt, H. H. H. W., Hofmann, F. & Stasch, J. P.) 309–339 (Springer, 2009).
Thoonen, R. et al. Cardiovascular and pharmacological implications of haem-deficient NO-unresponsive soluble guanylate cyclase knock-in mice. Nat. Commun. 6, 8482 (2015).
Ko, F. N., Wu, C. C., Kuo, S. C., Lee, F. Y. & Teng, C. M. YC-1, a novel activator of platelet guanylate cyclase. Blood 84, 4226–4233 (1994).
Friebe, A., Schultz, G. & Koesling, D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 15, 6863–6868 (1996).
Stasch, J. P. & Hobbs, A. J. in Handbook of Experimental Pharmacology Vol. 191 (eds Schmidt, H. H. H. W., Hofmann, F. & Stasch, J. P.) 277–308 (Springer, 2009).
Wunder, F. et al. A cell-based cGMP assay useful for ultra-high-throughput screening and identification of modulators of the nitric oxide/cGMP pathway. Anal. Biochem. 339, 104–112 (2005).
Rothkegel, C. et al. Identification of residues crucially involved in soluble guanylate cyclase activation. FEBS Lett. 580, 4205–4213 (2006).
Andersson, K. E. & Persson, K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J. Urol. 12, 274–280 (1994).
Iversen, H. H., Ehren, I., Gustafsson, L. E., Adolfsson, J. & Wiklund, N. P. Modulation of smooth muscle activity by nitric oxide in the human upper urinary tract. Urol. Res. 23, 391–394 (1995).
Ehren, I., Adolfsson, J. & Wiklund, N. P. Nitric oxide synthase activity in the human urogenital tract. Urol. Res. 22, 287–290 (1994).
Rajfer, J., Aronson, W. J., Bush, P. A., Dorey, F. J. & Ignarro, L. J. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N. Engl. J. Med. 326, 90–94 (1992).
Chertin, B., Rolle, U., Solari, V., Cascio, S. & Puri, P. The role of nitric oxide in bladder urothelial injury after bladder outlet obstruction. BJU Int. 94, 392–399 (2004).
Gillespie, J. I., Markerink-van Ittersum, M. & de Vente, J. Expression of neuronal nitric oxide synthase (nNOS) and nitric-oxide-induced changes in cGMP in the urothelial layer of the guinea pig bladder. Cell Tissue Res. 321, 341–351 (2005).
Gonzalez-Soriano, J. et al. Nitric oxide synthase in the external urethral sphincter of the sheep: immunohistochemical and functional study. J. Urol. 169, 1901–1906 (2003).
Otunctemur, A. et al. The comparison of GLUT-4 and nNOS expression in diabetic and non-diabetic patients with BPH/LUTS. Int. Urol. Nephrol. 47, 899–904 (2015).
Burnett, A. L. et al. Urinary bladder-urethral sphincter dysfunction in mice with targeted disruption of neuronal nitric oxide synthase models idiopathic voiding disorders in humans. Nat. Med. 3, 571–574 (1997).
Mamas, M. A., Reynard, J. M. & Brading, A. F. Nitric oxide and the lower urinary tract: current concepts, future prospects. Urology 61, 1079–1085 (2003).
Burnett, A. L. et al. Characterization and localization of nitric oxide synthase in the human prostate. Urology 45, 435–439 (1995).
Bloch, W. et al. Evidence for the involvement of endothelial nitric oxide synthase from smooth muscle cells in the erectile function of the human corpus cavernosum. Urol. Res. 26, 129–135 (1998).
Ghalayini, I. F. Nitric oxide-cyclic GMP pathway with some emphasis on cavernosal contractility. Int. J. Impot Res. 16, 459–469 (2004).
Mumtaz, F. H., Khan, M. A., Thompson, C. S., Morgan, R. J. & Mikhailidis, D. P. Nitric oxide in the lower urinary tract: physiology and pathological implications. BJU Int. 85, 611–613 (2000).
Fathian-Sabet, B. et al. Localization of constitutive nitric oxide synthase isoforms and the nitric oxide target enzyme soluble guanylyl cyclase in the human bladder. J. Urol. 165, 1724–1729 (2001).
Calmasini, F. B. et al. Implication of Rho-kinase and soluble guanylyl cyclase enzymes in prostate smooth muscle dysfunction in middle-aged rats. Neurourol. Urodyn. 36, 589–596 (2017).
Behrends, S., Steenpass, A., Porst, H. & Scholz, H. Expression of nitric oxide-sensitive guanylyl cyclase subunits in human corpus cavernosum. Biochem. Pharmacol. 59, 713–717 (2000).
Klotz, T. et al. Soluble guanylate cyclase and cGMP-dependent protein kinase I expression in the human corpus cavernosum. Int. J. Impot. Res. 12, 157–164 (2000).
Silva, F. H. et al. Prolonged therapy with the soluble guanylyl cyclase activator BAY 60–2770 restores the erectile function in obese mice. J. Sex. Med. 11, 2661–2670 (2014).
Muller, D., Mukhopadhyay, A. K., Davidoff, M. S. & Middendorff, R. Cyclic GMP signaling in rat urinary bladder, prostate, and epididymis: tissue-specific changes with aging and in response to Leydig cell depletion. Reproduction 142, 333–343 (2011).
Leiria, L. O. et al. The soluble guanylyl cyclase activator BAY 60–2770 ameliorates overactive bladder in obese mice. J. Urol. 191, 539–547 (2014).
Andersson, K. E., Garcia Pascual, A., Persson, K., Forman, A. & Tottrup, A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J. Urol. 147, 253–259 (1992).
Garcia-Pascual, A., Costa, G., Garcia-Sacristan, A. & Andersson, K. E. Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol. Scand. 141, 531–539 (1991).
Hashimoto, S., Kigoshi, S. & Muramatsu, I. Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. Eur. J. Pharmacol. 231, 209–214 (1993).
Werkstrom, V. et al. Factors involved in the relaxation of female pig urethra evoked by electrical field stimulation. Br. J. Pharmacol. 116, 1599–1604 (1995).
Masuda, H. et al. Involvement of accumulated endogenous NOS inhibitors and decreased NOS activity in the impaired neurogenic relaxation of the rabbit proximal urethra with ischaemia. Br. J. Pharmacol. 133, 97–106 (2001).
Persson, K. et al. Functional characteristics of urinary tract smooth muscles in mice lacking cGMP protein kinase type I. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1112–R1120 (2000).
Fraser, M. O. & Chancellor, M. B. Neural control of the urethra and development of pharmacotherapy for stress urinary incontinence. BJU Int. 91, 743–748 (2003).
James, M. J., Birmingham, A. T. & Hill, S. J. Partial mediation by nitric oxide of the relaxation of human isolated detrusor strips in response to electrical field stimulation. Br. J. Clin. Pharmacol. 35, 366–372 (1993).
Masuda, H. et al. Localization and role of nitric oxide synthase and endogenous nitric oxide synthase inhibitors in the rabbit lower urinary tract. J. Urol. 167, 2235–2240 (2002).
Andersson, K. E. & Arner, A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 84, 935–986 (2004).
Hayek, O. R. et al. Castration induces acute vasoconstriction of blood vessels in the rat prostate concomitant with a reduction of prostatic nitric oxide synthase activity. J. Urol. 162, 1527–1531 (1999).
Takeda, M. & Lepor, H. Nitric oxide synthase in dog urethra: a histochemical and pharmacological analysis. Br. J. Pharmacol. 116, 2517–2523 (1995).
Aikawa, K., Yokota, T., Okamura, H. & Yamaguchi, O. Endogenous nitric oxide-mediated relaxation and nitrinergic innervation in the rabbit prostate: the changes with aging. Prostate 48, 40–46 (2001).
Najbar-Kaszkiel, A. T., Di Iulio, J. L., Li, C. G. & Rand, M. J. Characterisation of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea pigs, rabbits and pigs. Eur. J. Pharmacol. 337, 251–258 (1997).
Takeda, M., Tang, R., Shapiro, E., Burnett, A. L. & Lepor, H. Effects of nitric oxide on human and canine prostates. Urology 45, 440–446 (1995).
Cirino, G., Fusco, F., Imbimbo, C. & Mirone, V. Pharmacology of erectile dysfunction in man. Pharmacol. Ther. 111, 400–423 (2006).
Rahnama'i, M. S., Uckert, S., Hohnen, R. & van Koeveringe, G. A. The role of phosphodiesterases in bladder pathophysiology. Nat. Rev. Urol. 10, 414–424 (2013).
Porst, H. et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur. Urol. 60, 1105–1113 (2011).
Maselli, G. et al. Tadalafil versus solifenacin for persistent storage symptoms after prostate surgery in patients with erectile dysfunction: a prospective randomized study. Int. J. Urol. 18, 515–520 (2011).
Gacci, M. et al. Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur. Urol. 70, 124–133 (2016).
Blanker, M. H. et al. Voided volumes: normal values and relation to lower urinary tract symptoms in elderly men, a community-based study. Urology 57, 1093–1099 (2001).
Shiri, R. et al. Erectile dysfunction influences the subsequent incidence of lower urinary tract symptoms and bother. Int. J. Impot. Res. 19, 317–320 (2007).
Brookes, S. T., Link, C. L., Donovan, J. L. & McKinlay, J. B. Relationship between lower urinary tract symptoms and erectile dysfunction: results from the Boston Area Community Health Survey. J. Urol. 179, 250–255 (2008).
Vignozzi, L., Gacci, M. & Maggi, M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat. Rev. Urol. 13, 108–119 (2016).
Parsons, J. K. et al. Obesity increases and physical activity decreases lower urinary tract symptom risk in older men: the Osteoporotic Fractures in Men study. Eur. Urol. 60, 1173–1180 (2011).
Penson, D. F., Munro, H. M., Signorello, L. B., Blot, W. J. & Fowke, J. H. Obesity, physical activity and lower urinary tract symptoms: results from the Southern Community Cohort Study. J. Urol. 186, 2316–2322 (2011).
Maserejian, N. N. et al. Treatment status and progression or regression of lower urinary tract symptoms in a general adult population sample. J. Urol. 191, 107–113 (2014).
Lee, R. K., Chung, D., Chughtai, B., Te, A. E. & Kaplan, S. A. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. 110, 540–545 (2012).
Daneshgari, F., Liu, G. & Hanna-Mitchell, A. T. Path of translational discovery of urological complications of obesity and diabetes. Am. J. Physiol. Renal Physiol. 312, F887–F896 (2017).
Nandeesha, H., Koner, B. C., Dorairajan, L. N. & Sen, S. K. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin. Chim. Acta 370, 89–93 (2006).
Vermeulen, A., Kaufman, J. M., Deslypere, J. P. & Thomas, G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J. Clin. Endocrinol. Metab. 76, 1140–1146 (1993).
Cohen, P. G. The role of estradiol in the maintenance of secondary hypogonadism in males in erectile dysfunction. Med. Hypotheses 50, 331–333 (1998).
Zumoff, B., Miller, L. K. & Strain, G. W. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism 52, 1126–1128 (2003).
Podlasek, C. A. et al. Translational Perspective on the Role of Testosterone in Sexual Function and Dysfunction. J. Sex. Med. 13, 1183–1198 (2016).
Mirone, V. et al. European Association of Urology Position Statement on the Role of the Urologist in the Management of Male Hypogonadism and Testosterone Therapy. Eur. Urol. 72, 164–167 (2017).
Snyder, P. J. et al. Effects of Testosterone Treatment in Older Men. N. Engl. J. Med. 374, 611–624 (2016).
Hackett, G. et al. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 118, 804–813 (2016).
Torimoto, K. et al. Urethral dysfunction in diabetic rats. J. Urol. 171, 1959–1964 (2004).
Daneshgari, F. et al. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1728–1735 (2006).
Saito, M. et al. Pharmacological properties, functional alterations and gene expression of muscarinic receptors in young and old type 2 Goto-Kakizaki diabetic rat bladders. J. Urol. 180, 2701–2705 (2008).
Nobe, K., Yamazaki, T., Tsumita, N., Hashimoto, T. & Honda, K. Glucose-dependent enhancement of diabetic bladder contraction is associated with a rho kinase-regulated protein kinase C pathway. J. Pharmacol. Exp. Ther. 328, 940–950 (2009).
Turner, W. H. & Brading, A. F. Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol. Ther. 75, 77–110 (1997).
Leiria, L. O. et al. Functional, morphological and molecular characterization of bladder dysfunction in streptozotocin-induced diabetic mice: evidence of a role for L-type voltage-operated Ca2+ channels. Br. J. Pharmacol. 163, 1276–1288 (2011).
Lee, W. C., Chien, C. T., Yu, H. J. & Lee, S. W. Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J. Urol. 179, 2470–2476 (2008).
Toque, H. A. et al. High-fat diet associated with obesity induces impairment of mouse corpus cavernosum responses. BJU Int. 107, 1628–1634 (2011).
Nunes, K. P., Teixeira, C. E., Priviero, F. B., Toque, H. A. & Webb, R. C. Beneficial effect of the soluble guanylyl cyclase stimulator BAY 41–2272 on impaired penile erection in db/db−/− type II diabetic and obese mice. J. Pharmacol. Exp. Ther. 353, 330–339 (2015).
Zhou, Z. et al. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol. Res. 111, 556–562 (2016).
Leiria, L. O. et al. Insulin relaxes bladder via PI3K/AKT/eNOS pathway activation in mucosa: unfolded protein response-dependent insulin resistance as a cause of obesity-associated overactive bladder. J. Physiol. 591, 2259–2273 (2013).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Yoshimura, K. et al. Prevalence of and risk factors for nocturia: analysis of a health screening program. Int. J. Urol. 11, 282–287 (2004).
Ponholzer, A., Temml, C., Wehrberger, C., Marszalek, M. & Madersbacher, S. The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur. Urol. 50, 581–586 (2006).
Yokoyama, O. et al. Nocturnal polyuria and hypertension in patients with lifestyle related diseases and overactive bladder. J. Urol. 197, 423–431 (2017).
Ramos-Filho, A. C. et al. Blockade of renin-angiotensin system prevents micturition dysfunction in renovascular hypertensive rats. Eur. J. Pharmacol. 738, 285–292 (2014).
Ribeiro, M. O., Antunes, E., de Nucci, G., Lovisolo, S. M. & Zatz, R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 20, 298–303 (1992).
Persson, K., Igawa, Y., Mattiasson, A. & Andersson, K. E. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br. J. Pharmacol. 107, 178–184 (1992).
Calmasini, F. B. et al. Increased Rho-kinase-mediated prostate contractions associated with impairment of beta-adrenergic-cAMP-signaling pathway by chronic nitric oxide deficiency. Eur. J. Pharmacol. 758, 24–30 (2015).
Claudino, M. A. et al. Long-term oral treatment with BAY 41–2272 ameliorates impaired corpus cavernosum relaxations in a nitric oxide-deficient rat model. BJU Int. 108, 116–122 (2011).
Teixeira, C. E., Priviero, F. B. & Webb, R. C. Effects of 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]pyrim idin-4-ylamine (BAY 41–2272) on smooth muscle tone, soluble guanylyl cyclase activity, and NADPH oxidase activity/expression in corpus cavernosum from wild-type, neuronal, and endothelial nitric-oxide synthase null mice. J. Pharmacol. Exp. Ther. 322, 1093–1102 (2007).
Hammarsten, J. & Peeker, R. Urological aspects of the metabolic syndrome. Nat. Rev. Urol. 8, 483–494 (2011).
Silva, F. H. et al. Superoxide anion production by NADPH oxidase plays a major role in erectile dysfunction in middle-aged rats: prevention by antioxidant therapy. J. Sex. Med. 10, 960–971 (2013).
Fibbi, B. et al. Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. J. Sex. Med. 7, 59–69 (2010).
Kalsi, J. S. et al. BAY41-2272, a novel nitric oxide independent soluble guanylate cyclase activator, relaxes human and rabbit corpus cavernosum in vitro. J. Urol. 169, 761–766 (2003).
Baracat, J. S. et al. Relaxing effects induced by the soluble guanylyl cyclase stimulator BAY 41–2272 in human and rabbit corpus cavernosum. Eur. J. Pharmacol. 477, 163–169 (2003).
Bischoff, E., Schramm, M., Straub, A., Feurer, A. & Stasch, J. P. BAY 41-2272: a stimulator of soluble guanylyl cyclase induces nitric oxide-dependent penile erection in vivo. Urology 61, 464–467 (2003).
Nimmegeers, S. et al. Role of the soluble guanylyl cyclase alpha1-subunit in mice corpus cavernosum smooth muscle relaxation. Int. J. Impot Res. 20, 278–284 (2008).
Lasker, G. F. et al. The sGC activator BAY 60–2770 has potent erectile activity in the rat. Am. J. Physiol. Heart Circ. Physiol. 304, H1670–H1679 (2013).
Stephens, R. S. et al. cGMP increases antioxidant function and attenuates oxidant cell death in mouse lung microvascular endothelial cells by a protein kinase G-dependent mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L323–L333 (2010).
Hoffmann, L. S. et al. Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation. Br. J. Pharmacol. 157, 781–795 (2009).
Martin, F. et al. Structure of cinaciguat (BAY 58–2667) bound to Nostoc H-NOX domain reveals insights into heme-mimetic activation of the soluble guanylyl cyclase. J. Biol. Chem. 285, 22651–22657 (2010).
Kumar, V. et al. Insights into BAY 60–2770 activation and S-nitrosylation-dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes. Biochemistry 52, 3601–3608 (2013).
McMurray, G., Casey, J. H. & Naylor, A. M. Animal models in urological disease and sexual dysfunction. Br. J. Pharmacol. 147 (Suppl. 2), S62–S79 (2006).
Schroder, A., Uvelius, B., Newgreen, D. & Andersson, K. E. Bladder overactivity in mice after 1 week of outlet obstruction. Mainly afferent dysfunction? J. Urol. 170, 1017–1021 (2003).
Hanno, P. M., Erickson, D., Moldwin, R. & Faraday, M. M. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol. 193, 1545–1553 (2015).
van de Merwe, J. P. et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur. Urol. 53, 60–67 (2008).
de Oliveira, M. G. et al. Activation of soluble guanylyl cyclase by BAY 58–2667 improves bladder function in cyclophosphamide-induced cystitis in mice. Am. J. Physiol. Renal Physiol. 311, F85–F93 (2016).
Condorelli, R. A. et al. Arterial erectile dysfunction: different severities of endothelial apoptosis between diabetic patients “responders” and “non responders” to sildenafil. Eur. J. Intern. Med. 24, 234–240 (2013).
Lizarte, F. S. et al. Chronic alcoholism associated with diabetes impairs erectile function in rats. BJU Int. 105, 1592–1597 (2010).
Christ, G. J. The penis as a vascular organ. The importance of corporal smooth muscle tone in the control of erection. Urol. Clin. North Am. 22, 727–745 (1995).
Fried, N. M. & Burnett, A. L. Novel methods for mapping the cavernous nerves during radical prostatectomy. Nat. Rev. Urol. 12, 451–460 (2015).
Kinsella, J. P., Neish, S. R., Shaffer, E. & Abman, S. H. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340, 819–820 (1992).
Kinsella, J. P. & Abman, S. H. Inhalational nitric oxide therapy for persistent pulmonary hypertension of the newborn. Pediatrics 91, 997–998 (1993).
Montani, D. et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol. Ther. 141, 172–119 (2014).
Beyer, C. et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFbeta signalling. Ann. Rheum. Dis. 74, 1408–1416 (2015).
Roehrborn, C. G., McVary, K. T., Elion-Mboussa, A. & Viktrup, L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J. Urol. 180, 1228–1234 (2008).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00855465 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00810693 (2016).
Galiè, N., Müller, K., Scalise, A.-V. & Grünig, E. Patent Plus: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur. Respir. J. 45, 1314–1322 (2008).
Acknowledgements
F.Z.M. and E.A. acknowledge FAPESP, CAPES, CNPq, and UNICAMP for research support.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the manuscript, made substantial contributions to discussion of content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mónica, F., Antunes, E. Stimulators and activators of soluble guanylate cyclase for urogenital disorders. Nat Rev Urol 15, 42–54 (2018). https://doi.org/10.1038/nrurol.2017.181
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.181
This article is cited by
-
Low-energy shock wave therapy ameliorates ischemic-induced overactive bladder in a rat model
Scientific Reports (2022)
-
Mechanism of traditional Chinese medicine in treating overactive bladder
International Urology and Nephrology (2022)
-
TMPRSS2-ERG activates NO-cGMP signaling in prostate cancer cells
Oncogene (2019)
-
Two Birds with One Stone: Regular Use of PDE5 Inhibitors for Treating Male Patients with Erectile Dysfunction and Cardiovascular Diseases
Cardiovascular Drugs and Therapy (2019)