Abstract
The lethal consequences of prostate cancer are related to its metastasis to other organ sites. Epithelial-to-mesenchymal transition (EMT) has received considerable attention as a conceptual paradigm to explain invasive and metastatic behavior during cancer progression. EMT is a normal physiologic process by which cells of epithelial origin convert into cells bearing mesenchymal characteristics. It has been proposed that EMT is co-opted by cancer cells during their metastatic dissemination from a primary organ to secondary sites, but the extent to which this recapitulates physiologic EMT remains uncertain. However, there is ample evidence that EMT-like states occur in, and may contribute to, prostate cancer progression and metastasis, and so has become a very active area of research. Here we review this evidence and explore recent studies that have aimed to better define the role and mechanisms of EMT in prostate cancer. While definitive evidence of something akin to physiologic EMT is still lacking in human prostate cancer, this area of research has nonetheless provided new avenues of investigation into the longstanding puzzles of metastasis, therapeutic resistance, and prognostic biomarkers.
Key Points
-
Epithelial-to-mesenchymal transition (EMT) is a normal physiologic process that involves lineage-specific, reversible transdifferentiation of epithelial cells in response to local inductive stimuli
-
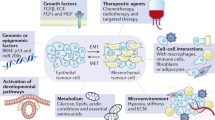
Prostate cancers exhibit EMT-like states, characterized by changes in the expression of various markers, such as E-cadherin and vimentin, which are associated with invasive behavior
-
Many mechanisms have been reported to produce EMT-like states in prostate cancer
-
The degree to which EMT-like states in prostate cancer result from processes similar to those that produce physiologic EMT is unclear
-
EMT-like states are associated with metastatic behavior and therapeutic resistance, so are potential targets for biomarker development or novel therapeutics
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Hay, E. D. Role of cell-matrix contacts in cell migration and epithelial-mesenchymal transformation. Cell Differ. Dev. 32, 367–375 (1990).
Bussemakers, M. J. et al. Decreased expression of E-cadherin in the progression of rat prostatic cancer. Cancer Res. 52, 2916–2922 (1992).
Thompson, E. W., Newgreen, D. F. & Tarin, D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 65, 5991–5995 (2005).
Tarin, D., Thompson, E. W. & Newgreen, D. F. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 65, 5996–6000 (2005).
Gleason, D. F. Classification of prostatic carcinomas. Cancer Chemother. Rep. 50, 125–128 (1966).
Randolph, T. L., Amin, M. B., Ro, J. Y. & Ayala, A. G. Histologic variants of adenocarcinoma and other carcinomas of prostate: pathologic criteria and clinical significance. Mod. Pathol. 10, 612–629 (1997).
McCarthy, R. P. et al. Molecular genetic evidence for different clonal origins of epithelial and stromal components of phyllodes tumor of the prostate. Am. J. Pathol. 165, 1395–1400 (2004).
Umbas, R. et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 52, 5104–5109 (1992).
Cheng, L., Nagabhushan, M., Pretlow, T. P., Amini, S. B. & Pretlow, T. G. Expression of E-cadherin in primary and metastatic prostate cancer. Am. J. Pathol. 148, 1375–1380 (1996).
Rubin, M. A. et al. E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum. Pathol. 32, 690–697 (2001).
De Marzo, A. M., Knudsen, B., Chan-Tack, K. & Epstein, J. I. E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology 53, 707–713 (1999).
Chaffer, C. L. et al. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 66, 11271–11278 (2006).
Berx, G. & van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect Biol. 1, a003129 (2009).
Tomita, K. et al. Cadherin switching in human prostate cancer progression. Cancer Res. 60, 3650–3654 (2000).
Rokhlin, O. W. & Cohen, M. B. Expression of cellular adhesion molecules on human prostate tumor cell lines. Prostate 26, 205–212 (1995).
Drake, J. M., Strohbehn, G., Bair, T. B., Moreland, J. G. & Henry, M. D. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol. Biol. Cell 20, 2207–2217 (2009).
Sethi, S., Macoska, J., Chen, W. & Sarkar, F. H. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am. J. Transl. Res. 3, 90–99 (2010).
Liao, C. P. et al. Mouse prostate cancer cell lines established from primary and post-castration recurrent tumors. Horm. Cancer 1, 44–54 (2010).
Tran, N. L., Nagle, R. B., Cress, A. E. & Heimark, R. L. N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion withStromal cells. Am. J. Pathol. 155, 787–798 (1999).
Chunthapong, J. et al. Dual roles of E-cadherin in prostate cancer invasion. J. Cell. Biochem. 91, 649–661 (2004).
Zhau, H. E. et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin. Exp. Metastasis 25, 601–610 (2008).
Drake, J. M. et al. ZEB1 coordinately regulates laminin-332 and β4 integrin expression altering the invasive phenotype of prostate cancer cells. J. Biol. Chem. 285, 33940–33948 (2010).
Harma, V. et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS ONE 5, e10431.
Chu, J. H., Yu, S., Hayward, S. W. & Chan, F. L. Development of a three-dimensional culture model of prostatic epithelial cells and its use for the study of epithelial-mesenchymal transition and inhibition of PI3K pathway in prostate cancer. Prostate 69, 428–442 (2009).
Yates, C. C., Shepard, C. R., Stolz, D. B. & Wells, A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br. J. Cancer 96, 1246–1252 (2007).
Mani, S. A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Polyak, K. & Weinberg, R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 (2009).
Yu, J. et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 (2010).
Wang, Z. A. & Shen, M. M. Revisiting the concept of cancer stem cells in prostate cancer. Oncogene 30, 1261–1271 (2011).
Goldstein, A. S., Stoyanova, T. & Witte, O. N. Primitive origins of prostate cancer: in vivo evidence for prostate-regenerating cells and prostate cancer-initiating cells. Mol. Oncol. 4, 385–396 (2010).
Kong, D. et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE 5, e12445 (2010).
Klarmann, G. J. et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis 26, 433–446 (2009).
Ke, X. S. et al. Epithelial to mesenchymal transition of a primary prostate cell line with switches of cell adhesion modules but without malignant transformation. PLoS ONE 3, e3368 (2008).
Klymkowsky, M. W. & Savagner, P. Epithelial–mesenchymal transition: a cancer researcher's conceptual friend and foe. Am. J. Pathol. 174, 1588–1593 (2009).
Gan, Y. et al. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29, 4947–4958 (2010).
Wells, C. M., Ahmed, T., Masters, J. R. & Jones, G. E. Rho family GTPases are activated during HGF-stimulated prostate cancer-cell scattering. Cell Motil. Cytoskeleton 62, 180–194 (2005).
Graham, T. R. et al. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 68, 2479–2488 (2008).
Kong, D. et al. Platelet-derived growth factor-D overexpression contributes to epithelial–mesenchymal transition of PC3 prostate cancer cells. Stem Cells 26, 1425–1435 (2008).
Giri, D., Ropiquet, F. & Ittmann, M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin. Cancer Res. 5, 1063–1071 (1999).
Acevedo, V. D. et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell 12, 559–571 (2007).
Sakai, D., Suzuki, T., Osumi, N. & Wakamatsu, Y. Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development 133, 1323–1333 (2006).
Cao, J. et al. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J. Biol. Chem. 283, 6232–6240 (2008).
Rashid, M. G. et al. Posttranslational truncation and inactivation of human E-cadherin distinguishes prostate cancer from matched normal prostate. Cancer Res. 61, 489–492 (2001).
Massagué, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Zhang, Q. et al. Nuclear factor-κB-mediated transforming growth factor-β-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin. Cancer Res. 15, 3557–3567 (2009).
Huber, M. A. et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569–581 (2004).
Pu, H. et al. Dysfunctional transforming growth factor-β receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res. 69, 7366–7374 (2009).
Tu, W. H. et al. The loss of TGF-β signaling promotes prostate cancer metastasis. Neoplasia 5, 267–277 (2003).
Giannoni, E. et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956 (2010).
Ao, M., Williams, K., Bhowmick, N. A. & Hayward, S. W. Transforming growth factor-β promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 66, 8007–8016 (2006).
Tuxhorn, J. A. et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 8, 2912–2923 (2002).
Bokobza, S. M., Ye, L., Kynaston, H. & Jiang, W. G. Growth and differentiation factor 9 (GDF-9) induces epithelial-mesenchymal transition in prostate cancer cells. Mol. Cell. Biochem. 349, 33–40 (2011).
Buijs, J. T. et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am. J. Pathol. 171, 1047–1057 (2007).
Imai, T. et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am. J. Pathol. 163, 1437–1447 (2003).
Mak, P. et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated Snail nuclear localization: implications for Gleason grading. Cancer Cell 17, 319–332 (2010).
Gonzalez-Moreno, O. et al. VEGF elicits epithelial-mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Exp. Cell Res. 316, 554–567 (2010).
Xie, D. et al. DAB2IP coordinates both PI3K–Akt and ASK1 pathways for cell survival and apoptosis. Proc. Natl Acad. Sci. USA 106, 19878–19883 (2009).
Wang, Z. et al. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC-2/DAB2. J. Biol. Chem. 277, 12622–12631 (2002).
Chen, H., Toyooka, S., Gazdar, A. F. & Hsieh, J. T. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J. Biol. Chem. 278, 3121–3130 (2003).
Chen, H., Tu, S. W. & Hsieh, J. T. Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J. Biol. Chem. 280, 22437–22444 (2005).
Xie, D. et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc. Natl Acad. Sci. USA 107, 2485–2490 (2010).
Min, J. et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-κB. Nat. Med. 16, 286–294 (2010).
Varambally, S. et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002).
Zi, X. et al. Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 65, 9762–9770 (2005).
Yee, D. S. et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol. Cancer 9, 162 (2010).
Gupta, S. et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 70, 6735–6745 (2010).
Du, C., Zhang, C., Hassan, S., Biswas, M. H. & Balaji, K. C. Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of snail. Cancer Res. 70, 7810–7819 (2010).
Shah, G. V., Muralidharan, A., Gokulgandhi, M., Soan, K. & Thomas, S. Cadherin switching and activation of β-catenin signaling underlie proinvasive actions of calcitonin-calcitonin receptor axis in prostate cancer. J. Biol. Chem. 284, 1018–1030 (2009).
Cai, H., Babic, I., Wei, X., Huang, J. & Witte, O. N. Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res. 71, 862–872 (2011).
Zhu, M. L. & Kyprianou, N. Role of androgens and the androgen receptor in epithelial–mesenchymal transition and invasion of prostate cancer cells. FASEB J. 24, 769–777 (2010).
Gu, X. et al. Reduced PDEF expression increases invasion and expression of mesenchymal genes in prostate cancer cells. Cancer Res. 67, 4219–4226 (2007).
Beach, S. et al. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene 27, 2243–2248 (2008).
Kwok, W. K. et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 65, 5153–5162 (2005).
Alexander, N. R. et al. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res. 66, 3365–3369 (2006).
Kogan-Sakin, I. et al. Mutant p53R175H upregulates Twist1 expression and promotes epithelial-mesenchymal transition in immortalized prostate cells. Cell Death Differ. 18, 271–281 (2011).
Li, L. C. et al. Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. J. Urol. 166, 705–709 (2001).
Saha, B. et al. Unmethylated E-cadherin gene expression is significantly associated with metastatic human prostate cancer cells in bone. Prostate 68, 1681–1688 (2008).
Cao, Q. et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27, 7274–7284 (2008).
Herranz, N. et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol. Cell. Biol. 28, 4772–4781 (2008).
Lukacs, R. U., Memarzadeh, S., Wu, H. & Witte, O. N. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 7, 682–693 (2010).
van Leenders, G. J. et al. Polycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features. Eur. Urol. 52, 455–463 (2007).
Gregory, P. A. et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 10, 593–601 (2008).
Korpal, M., Lee, E. S., Hu, G. & Kang, Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 283, 14910–14914 (2008).
Park, S. M., Gaur, A. B., Lengyel, E. & Peter, M. E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 22, 894–907 (2008).
Gregory, P. A. et al. An autocrine TGF-β/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol. Biol. Cell doi: 10.1091/mbc.E11-02-0103.
Kong, D. et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells 27, 1712–1721 (2009).
Saini, S. et al. Regulatory role of miR-203 in prostate cancer progression and metastasis. Clin. Cancer Res. doi: 10.1158/1078-0432.CCR-10-2619.
Gandellini, P. et al. miR-205 exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cɛ. Cancer Res. 69, 2287–2295 (2009).
Varambally, S. et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322, 1695–1699 (2008).
Cao, P. et al. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1α/HIF-1β. Mol. Cancer 9, 108 (2010).
Suyama, K., Shapiro, I., Guttman, M. & Hazan, R. B. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2, 301–314 (2002).
Jennbacken, K. et al. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr. Relat. Cancer 17, 469–479 (2010).
Tanaka, H. et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat. Med. 16, 1414–1420 (2010).
Steffan, J. J., Williams, B. C., Welbourne, T. & Cardelli, J. A. HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+–H+ exchangers. J. Cell Sci. 123, 1151–1159 (2010).
Singh, S. et al. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 63, 2306–2311 (2003).
Tsuji, T. et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 68, 10377–10386 (2008).
Tran, N. L., Adams, D. G., Vaillancourt, R. R. & Heimark, R. L. Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J. Biol. Chem. 277, 32905–32914 (2002).
Escriva, M. et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol. Cell. Biol. 28, 1528–1540 (2008).
Crea, F. et al. BMI1 silencing enhances docetaxel activity and impairs antioxidant response in prostate cancer. Int. J. Cancer 128, 1946–1954 (2011).
Odero-Marah, V. A. et al. Receptor activator of NF-κB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 18, 858–870 (2008).
Yuen, H. F. et al. TWIST modulates prostate cancer cell-mediated bone cell activity and is upregulated by osteogenic induction. Carcinogenesis 29, 1509–1518 (2008).
Ansieau, S. et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14, 79–89 (2008).
Yu, J. et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 67, 10657–10663 (2007).
Ruijter, E. et al. Heterogeneous expression of E-cadherin and p53 in prostate cancer: clinical implications. BIOMED-II Markers for Prostate Cancer Study Group. Mod. Pathol. 11, 276–281 (1998).
Pantel, K. & Alix-Panabières, C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol. Med. 16, 398–406 (2010).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Singh, R. P., Raina, K., Sharma, G. & Agarwal, R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin. Cancer Res. 14, 7773–7780 (2008).
Zhang, L. L. et al. A novel anti-cancer effect of genistein: reversal of epithelial mesenchymal transition in prostate cancer cells. Acta Pharmacol. Sin. 29, 1060–1068 (2008).
Wu, K. et al. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol. Rep. 23, 1545–1552 (2010).
Baritaki, S. et al. Mechanisms of nitric oxide-mediated inhibition of EMT in cancer: inhibition of the metastasis-inducer Snail and induction of the metastasis-suppressor RKIP. Cell Cycle 9, 4931–4940 (2010).
Baritaki, S. et al. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene 28, 3573–3585 (2009).
Lenferink, A. E. et al. Transcriptome profiling of a TGF-β-induced epithelial-to-mesenchymal transition reveals extracellular clusterin as a target for therapeutic antibodies. Oncogene 29, 831–844 (2010).
Umbas, R. et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 54, 3929–3933 (1994).
Lang, S. H. et al. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate 52, 253–263 (2002).
Jaggi, M. et al. N-cadherin switching occurs in high Gleason grade prostate cancer. Prostate 66, 193–199 (2006).
Kallakury, B. V., Sheehan, C. E. & Ross, J. S. Co-downregulation of cell adhesion proteins α- and β-catenins, p120CTN, E-cadherin, and CD44 in prostatic adenocarcinomas. Hum. Pathol. 32, 849–855 (2001).
Acknowledgements
We would like to thank members of the Henry laboratory and Dr. Christopher Stipp for critical reading of the manuscript, and Dr. Beatrice Knudsen for sharing unpublished results. Work related to this topic in the Henry laboratory has been supported by NIH grant RO1 CA130916 and DOD grant W81XWH-10-1-0313. We apologize to colleagues whose work we were unable to cite due to space constraints.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to researching data, discussing the content, writing the article and performing review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nauseef, J., Henry, M. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle?. Nat Rev Urol 8, 428–439 (2011). https://doi.org/10.1038/nrurol.2011.85
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.85
This article is cited by
-
The role of GCNT1 mediated O-glycosylation in aggressive prostate cancer
Scientific Reports (2023)
-
Single-cell analysis reveals androgen receptor regulates the ER-to-Golgi trafficking pathway with CREB3L2 to drive prostate cancer progression
Oncogene (2021)
-
Sequential Ras/MAPK and PI3K/AKT/mTOR pathways recruitment drives basal extrusion in the prostate-like gland of Drosophila
Nature Communications (2020)
-
Distinct roles of Dlk1 isoforms in bi-potential differentiation of hepatic stem cells
Stem Cell Research & Therapy (2019)
-
The p21-activated kinase 4-Slug transcription factor axis promotes epithelial−mesenchymal transition and worsens prognosis in prostate cancer
Oncogene (2018)