Abstract
The Wnt signalling cascades have essential roles in development, growth and homeostasis of joints and the skeleton. Progress in basic research, particularly relating to our understanding of intracellular signalling cascades and fine regulation of receptor activation in the extracellular space, has provided novel insights into the roles of Wnt signalling in chronic arthritis. Cartilage and bone homeostasis require finely tuned Wnt signalling; both activation and suppression of the Wnt–β-catenin cascade can lead to osteoarthritis in rodent models. Genetic associations with the Wnt antagonist encoded by FRZB and the transcriptional regulator encoded by Dot1l with osteoarthritis further corroborate the essential part played by Wnts in the joint. In rheumatoid arthritis, inhibition of Wnt signalling has a role in the persistence of bone erosions, whereas Wnts have been associated with the ankylosing phenotype in spondyloarthritis. Together, these observations identify the Wnt pathway as an attractive target for therapeutic intervention; however, the complexity of the Wnt signalling cascades and the potential secondary effects of drug interventions targeting them highlight the need for further research and suggest that our understanding of this exciting pathway is still in its infancy.
Key Points
-
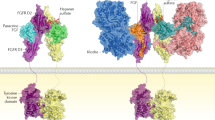
Wnts are lipid-modified glycoproteins that activate a variety of signalling pathways in an autocrine or paracrine manner; target cell activation is dependent on the formation of complex signalling centres
-
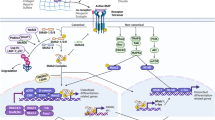
Wnts are essential for articular cartilage and bone homeostasis, but excessive Wnt signalling is associated with loss of the stable articular chondrocyte phenotype and can lead to cartilage damage
-
In rheumatoid arthritis, healing of erosions can be inhibited by elevated local concentrations of Wnt antagonists such as Dickkopf-related protein 1 and secreted frizzled-related protein 1
-
Wnts are associated with ankylosis in spondyloarthritis, and data from developmental biology suggest that specific Wnts stimulate direct bone formation and not the initial steps in endochondral ossification
-
Various Wnt antagonists have been proposed as biomarkers in patients with spondyloarthritis, but further validation is required
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clevers, H & Nusse, R. Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Kadowaki, T., Wilder, E., Klingensmith, J., Zachary, K. & Perrimon, N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 10, 3116–3128 (1996).
Bänziger, C. et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 (2006).
Tabata, T & Takei, Y. Morphogens, their identification and regulation. Development 131, 703–712 (2004).
Mii, Y & Taira, M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 136, 4083–4088 (2009).
Mii, Y & Taira, M. Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev. Growth Differ. 53, 911–923 (2011).
Bovolenta, P., Esteve, P., Ruiz, J. M., Cisneros, E. & Lopez-Rios, J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121, 737–746 (2008).
Sugimura, R & Li, L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res. C Embryo Today 90, 243–256 (2010).
Li, V. S. et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149, 1245–1256 (2012).
Wallingford, J. B. & Mitchell, B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 25, 201–213 (2011).
Clark, C. E., Nourse, C. C. & Cooper, H. M. The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals 20, 202–220 (2012).
Minami, Y., Oishi, I., Endo, M. & Nishita M. ROR-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev. Dyn. 239, 1–15 (2010).
Matthews, H. K. et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 135, 1771–1780 (2008).
Cruciat, C. M. & Niehrs, C. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. http://dx.doi.org/10.1101/cshperspect.a015081.
Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779 (2012).
Leucht, P., Minear, S., Ten Berge, D., Nusse, R. & Helms, J. A. Translating insights from development into regenerative medicine: the function of Wnts in bone biology. Semin. Cell Dev. Biol. 19, 434–443 (2008).
Monroe, D. G., McGee-Lawrence, M. E., Oursler, M. J. & Westendorf, J. J. Update on Wnt signaling in bone cell biology and bone disease. Gene 492, 1–18 (2012).
Lefebvre, V. & Bhattaram, P. Vertebrate skeletogenesis. Curr. Top. Dev. Biol. 90, 291–317 (2010).
Barrow, J. R. et al. Ectodermal Wnt3/β-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 17, 394–409 (2003).
Kawakami, Y. et al. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell 104, 891–900 (2001).
Nam, J. S. et al. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev. Biol. 311, 124–135 (2007).
Gros, J. et al. Wnt5a/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr. Biol. 20, 1993–2002 (2010).
Rudnicki, J. A. & Brown, A. M. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev. Biol. 185, 104–118 (1997).
Witte, F., Dokas, J., Neuendorf, F., Mundlos, S. & Stricker, S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr. Patterns 9, 215–223 (2009).
Day, T. F., Guo, X., Garrett-Beal, L. & Yang, Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750 (2005).
Akiyama, H. et al. Interactions between SOX9 and β-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087 (2004).
Hill, T. P., Später, D., Taketo, M. M., Birchmeier, W. & Hartmann, C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell 8, 727–738 (2005).
Church, V., Nohno, T., Linker, C., Marcelle, C. & Francis-West, P. Wnt regulation of chondrocyte differentiation. J. Cell Sci. 115, 4809–4818 (2002).
Dong, Y. F., Soung do, Y., Schwarz, E. M., O'Keefe, R. J. & Drissi, H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J. Cell. Physiol. 208, 77–86 (2006).
Guo, X., Mak, K. K., Taketo, M. M. & Yang, Y. The Wnt/β-catenin pathway interacts differentially with PTHrP signaling to control chondrocyte hypertrophy and final maturation. PLoS ONE 4, e6067 (2009).
Tamamura, Y. et al. Developmental regulation of Wnt/β-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 280, 19185–19195 (2005).
Dao, D. Y. et al. Cartilage-specific β-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J. Bone Miner. Res. 27, 1680–1694 (2012).
Bradley, E. W. & Drissi, M. H. Wnt5a regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-κB pathways. Mol. Endocrinol. 24, 1581–1593 (2010).
Bradley, E. W. & Drissi, M. H. Wnt5b regulates mesenchymal cell aggregation and chondrocyte differentiation through the planar cell polarity pathway. J. Cell. Physiol. 226, 1683–1693 (2011).
Hu, H. et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132, 49–60 (2005).
Hartmann, C. & Tabin, C. J. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 104, 341–351 (2001).
Später, D., Hill, T. P., Gruber, M. & Hartmann, C. Role of canonical Wnt-signalling in joint formation. Eur. Cell. Mater. 12, 71–80 (2006).
Später, D. et al. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 133, 3039–3049 (2006).
Li, X. et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 280, 19883–19887 (2005).
Robling, A. G. et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 (2008).
Baker-Lepain, J. C. et al. Variant alleles of the Wnt antagonist FRZB are determinants of hip shape and modify the relationship between hip shape and osteoarthritis. Arthritis Rheum. 64, 1457–1465 (2012).
Loughlin, J. et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc. Natl Acad. Sci. USA 101, 9757–9762 (2004).
Lane, N. E. et al. Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum. 54, 1246–1254 (2006).
Min, J. L. et al. Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum. 52, 1077–1080 (2005).
Evangelou, E. et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 60, 1710–1721 (2009).
Castaño Betancourt, M. C. et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc. Natl Acad. Sci. USA 109, 8218–8223 (2012).
Mahmoudi, T. et al. The leukemia-associated Mllt10/Af10-Dot1l are TCF4/β-catenin coactivators essential for intestinal homeostasis. PLoS Biol. 8, e1000539 (2010).
Dell'accio, F., De Bari, C., Eltawil, N. M., Vanhummelen, P. & Pitzalis C. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 58, 1410–1421 (2008).
Blom, A. B. et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 60, 501–512 (2009).
Sen, M. et al. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 97, 2791–2796 (2000).
Nakamura, Y., Nawata, M. & Wakitani, S. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am. J. Pathol. 167, 97–105 (2005).
Imai, K. et al. Differential expression of Wnts and FRPs in the synovium of rheumatoid arthritis and osteoarthritis. Biochem. Biophys. Res. Commun. 345, 1615–1620 (2006).
Sen, M. et al. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum. 46, 2867–2877 (2002).
Sen, M., Chamorro, M., Reifert, J., Corr, M. & Carson, D. A. Blockade of Wnt-5a/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 44, 772–781 (2001).
Zhu, M. et al. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 58, 2053–2064 (2008).
Zhu, M. et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J. Bone Miner. Res. 24, 12–21 (2009).
Yuasa, T. et al. Transient activation of Wnt/β-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am. J. Pathol. 175, 1993–2003 (2009).
Lodewyckx, L., Luyten, F. P. & Lories R. J. Genetic deletion of low-density lipoprotein receptor-related protein 5 increases cartilage degradation in instability-induced osteoarthritis. Rheumatology (Oxford) 51, 1973–1978 (2012).
Lories, R. J. et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 56, 4095–4103 (2007).
Miclea, R. L. et al. Inhibition of GSK3β in cartilage induces osteoarthritic features through activation of the canonical Wnt signaling pathway. Osteoarthritis Cartilage 19, 1363–1372 (2011).
Weng, L. H., Wang, C. J., Ko, J. Y., Sun, Y. C. & Wang, F. S. Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum. 62, 1393–1402 (2010).
Oh, H., Chun, C. H. & Chun, J. S. Dkk-1 expression in chondrocytes inhibits experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 64, 2568–2578 (2012).
Leijten, J. C. et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 64, 3302–3012 (2012).
Lodewyckx, L., Cailotto, F., Thysen, S., Luyten, F. P. & Lories, R. J. Tight regulation of wingless-type signaling in the articular cartilage–subchondral bone biomechanical unit: transcriptomics in Frzb-knockout mice. Arthritis Res. Ther. 14, R16 (2012).
Nalesso, G. et al. Wnt-3a modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J. Cell Biol. 193, 551–564 (2011).
Cailotto, F., Sebillaud, S., Netter, P., Jouzeau, J. Y. & Bianchi, A. The inorganic pyrophosphate transporter ANK preserves the differentiated phenotype of articular chondrocyte. J. Biol. Chem. 285, 10572–10582 (2010).
Ma, B., van Blitterswijk, C. A. & Karperien, M. A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 64, 2589–2600 (2012).
Matzelle, M. M. et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 64, 1540–1550 (2012).
Walsh, N. C. et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J. Bone Miner. Res. 24, 1572–1585 (2009).
Li, X. et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 37, 945–952 (2005).
de Rooy, D. P. et al. Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-202184.
Glass, D. A. 2nd et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 (2005).
Diarra, D. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163 (2007).
Maiti, G., Naskar, D. & Sen, M. The Wingless homolog Wnt5a stimulates phagocytosis but not bacterial killing. Proc. Natl Acad. Sci. USA 109, 16600–16605 (2012).
Kim, J. et al. Wnt5a induces endothelial inflammation via β-catenin-independent signaling. J. Immunol. 185, 1274–1282 (2010).
Maeda, K. et al. Wnt5a–ROR2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 18, 405–412 (2012).
Lee, Y. S. et al. The Wnt inhibitor secreted frizzled-related protein 1 (sFRP1) promotes human TH17 differentiation. Eur. J. Immunol. 42, 2564–2573 (2012).
Dougados, M. & Baeten, D. Spondyloarthritis. Lancet 377, 2127–2137 (2011).
Lories, R. J., Luyten, F. P. & de Vlam, K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res. Ther. 11, 221 (2009).
Machado, P. et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann. Rheum. Dis. 69, 1465–1470 (2010).
Wanders, A. et al. Nonsteroidal anti-inflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 52, 1756–1765 (2005).
Poddubnyy, D. et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann. Rheum. Dis. 71, 1616–1622 (2012).
van der Heijde, D. et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 58, 1324–1331 (2008).
Uderhardt, S. et al. Blockade of Dickkopf (Dkk)-1 induces fusion of sacroiliac joints. Ann. Rheum. Dis. 69, 592–597 (2010).
Haynes, K. R. et al. Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors. Arthritis Res. Ther. 14, R253 (2012).
Heiland, G. R. et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann. Rheum. Dis. 71, 572–574 (2012).
Daoussis, D. et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 62, 150–158 (2010).
Appel, H. et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 60, 3257–3262 (2009).
Saad, C. G. et al. Low sclerostin levels: a predictive marker of persistent inflammation in ankylosing spondylitis during anti-tumor necrosis factor therapy? Arthritis Res. Ther. 14, R216 (2012).
Lane, N. E. et al. Wnt signaling antagonists are potential prognostic biomarkers for the progression of radiographic hip osteoarthritis in elderly Caucasian women. Arthritis Rheum. 56, 3319–3325 (2007).
Padhi, D. A. et al. The effects of multiple doses of sclerostin antibody AMG 785 in healthy men and postmenopausal women with low bone mass [abstract OP0044]. Ann. Rheum. Dis. 71 (Suppl. 3), 67–68 (2012).
Andrade, A. C., Nilsson, O., Barnes, K. M. & Baron, J. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone 40, 1361–1369 (2007).
Kengaku, M. et al. Distinct Wnt pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science 280, 1274–1277 (1998).
Guo, X. et al. Wnt/β-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 18, 2404–2417 (2004).
Hartmann, C. & Tabin, C. J. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 127, 3141–3159 (2000).
Yang, Y., Topol, L., Lee, H. & Wu, J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 130, 1003–1015 (2003).
Pasold, J. et al. Reduced expression of Sfrp1 during chondrogenesis and in articular chondrocytes correlates with osteoarthritis in STR/ort mice. Exp. Cell Res. http://dx.doi.org/10.1016/j.yexcr.2012.12.012.
Ladher, R. K. et al. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev. Biol. 218, 183–198 (2000).
Kusu, N. et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J. Biol. Chem. 278, 24113–24117 (2003).
Chan, B. Y. et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage 19, 874–885 (2011).
Roudier, M. et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum. http://dx.doi.org/10.1002/art.37802.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to each stage of the preparation of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lories, R., Corr, M. & Lane, N. To Wnt or not to Wnt: the bone and joint health dilemma. Nat Rev Rheumatol 9, 328–339 (2013). https://doi.org/10.1038/nrrheum.2013.25
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.25
This article is cited by
-
WTAP-mediated m6A modification of FRZB triggers the inflammatory response via the Wnt signaling pathway in osteoarthritis
Experimental & Molecular Medicine (2024)
-
Decreased expression of GEM in osteoarthritis cartilage regulates chondrogenic differentiation via Wnt/β-catenin signaling
Journal of Orthopaedic Surgery and Research (2023)
-
Periostin: an emerging activator of multiple signaling pathways
Journal of Cell Communication and Signaling (2022)
-
New Directions in the Development of Pharmacotherapy for Osteoarthrosis Based on Modern Concepts of the Disease Pathogenesis (A Review)
Pharmaceutical Chemistry Journal (2022)
-
WNT16 elevation induced cell senescence of osteoblasts in ankylosing spondylitis
Arthritis Research & Therapy (2021)