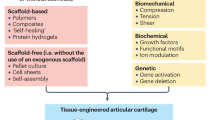
Abstract
Joint destruction occurs in both osteoarthritis and rheumatoid arthritis. Even in the era of biologic agents, this destruction can be delayed but not averted. As cartilage has limited ability to self-regenerate, joint arthroplasty is required. Here, we outline current tissue engineering procedures (including autologous chondrocyte implantation and in situ mesenchymal stem cell recruitment) that are routinely applied for the regenerative treatment of injured or early osteoarthritic cartilage. Potential future regenerative therapies, including administration of multipotent or pluripotent stem cells, are also discussed. In the future, cell-free, material-based (for cartilage lesions) or cell-free, factor-based (for osteoarthritic cartilage) therapies to facilitate the recruitment of repair cells and improve cartilage metabolism are likely to become more important. Moreover, delivery of anti-inflammatory factors or immunomodulatory cells could be a regenerative treatment option for rheumatoid arthritis. Tissue engineering faces a crucial phase to translate products into clinical routine and the regulatory framework for cell-based products in particular is an important issue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Grande, D. A., Pitman, M. I., Peterson, L., Menche, D. & Klein, M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J. Orthop. Res. 7, 208–218 (1989).
Daher, R. J., Chahine, N. O., Greenberg, A. S., Sgaglione, N. A. & Grande, D. A. New methods to diagnose and treat cartilage degeneration. Nat. Rev. Rheumatol. 5, 599–607 (2009).
Ossendorf, C. et al. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res. Ther. 9, R41 (2007).
Kon, E. et al. Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Eur. J. Radiol. 79, 382–388 (2011).
Behrens, P., Bitter, T., Kurz, B. & Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 13, 194–202 (2006).
Sittinger, M. & Burmester, G. R. Can engineered cartilage transplants be used for treating rheumatic diseases? Nat. Clin. Pract. Rheumatol. 2, 172–173 (2006).
Peterson, L., Vasiliadis, H. S., Brittberg, M. & Lindahl, A. Autologous chondrocyte implantation: a long-term follow-up. Am. J. Sports Med. 38, 1117–1124 (2010).
Knutsen, G. et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J. Bone Joint Surg. Am. 89, 2105–2112 (2007).
Saris, D. B. et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 36, 235–246 (2008).
Kreuz, P. C. et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am. J. Sports Med. 39, 1697–1705 (2011).
Benthien, J. P., Schwaninger, M. & Behrens, P. We do not have evidence based methods for the treatment of cartilage defects in the knee. Knee Surg. Sports Traumatol. Arthrosc. 19, 543–552 (2011).
Bartlett, W. et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J. Bone Joint Surg. Br. 87, 640–645 (2005).
Dehne, T., Karlsson, C., Ringe, J., Sittinger, M. & Lindahl, A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res. Ther. 11, R133 (2009).
Hollander, A. P. et al. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 12, 1787–1798 (2006).
Kreuz, P. C., Muller, S., Ossendorf, C., Kaps, C. & Erggelet, C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Res. Ther. 11, R33 (2009).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Jorgensen, C. & Noel, D. Mesenchymal stem cells in osteoarticular diseases. Regen. Med. 6, 44–51 (2011).
Murphy, J. M., Fink, D. J., Hunziker, E. B. & Barry, F. P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48, 3464–3474 (2003).
Davatchi, F., Abdollahi, B. S., Mohyeddin, M., Shahram, F. & Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 14, 211–215 (2011).
Nejadnik, H., Hui, J. H., Feng Choong, E. P., Tai, B. C. & Lee, E. H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am. J. Sports Med. 38, 1110–1116 (2010).
Kuroda, R. et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage 15, 226–231 (2007).
Wakitani, S. et al. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell. Transplant. 13, 595–600 (2004).
Wakitani, S. et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage 10, 199–206 (2002).
Kafienah, W. et al. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum. 56, 177–187 (2007).
Ringe, J. & Sittinger, M. Tissue engineering in the rheumatic diseases. Arthritis Res. Ther. 11, 211 (2009).
Mohal, J. S., Tailor, H. D. & Khan, W. S. Sources of adult mesenchymal stem cells and their applicability for musculoskeletal applications. Curr. Stem Cell Res. Ther. 7, 103–109 (2012).
Pei, M., He, F. & Vunjak-Novakovic, G. Synovium-derived stem cell-based chondrogenesis. Differentiation 76, 1044–1056 (2008).
De Bari, C., Dell'Accio, F. & Luyten, F. P. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 50, 142–150 (2004).
Kim, M. J. et al. Generation of human induced pluripotent stem cells from osteoarthritis patient-derived synovial cells. Arthritis Rheum. 63, 3010–3021 (2011).
Toh, W. S., Lee, E. H. & Cao, T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev. 7, 544–559 (2011).
Benthien, J. P. & Behrens, P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg. Sports Traumatol. Arthrosc. 19, 1316–1319 (2011).
Kusano, T. et al. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg. Sports Traumatol. Arthrosc. http://dx.doi.org/10.1007/s00167-011-1840-1842.
Erggelet, C. et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J. Orthop. Res. 27, 1353–1360 (2009).
Patrascu, J. M., Freymann, U., Kaps, C. & Poenaru, D. V. Repair of a post-traumatic cartilage defect with a cell-free polymer-based cartilage implant: a follow-up at two years by MRI and histological review. J. Bone Joint Surg. Br. 92, 1160–1163 (2010).
Siclari, A., Mascaro, G., Gentili, C., Cancedda, R. & Boux, E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin. Orthop. Relat. Res. 470, 910–919 (2012).
Pretzel, D. et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res. Ther. 13, R64 (2011).
Stich, S. et al. Gene expression profiling of human mesenchymal stem cells chemotactically induced with CXCL12. Cell Tissue Res. 336, 225–236 (2009).
Kruger, J. P. et al. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J. Orthop. Res. 30, 845–852 (2011).
Fiedler, J., Roderer, G., Gunther, K. P. & Brenner, R. E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell. Biochem. 87, 305–312 (2002).
Endres, M. et al. Synovial fluid recruits human mesenchymal progenitors from subchondral spongious bone marrow. J. Orthop. Res. 25, 1299–1307 (2007).
Kalwitz, G. et al. Chemokine profile of human serum from whole blood: migratory effects of CXCL-10 and CXCL-11 on human mesenchymal stem cells. Connect. Tissue Res. 51, 113–122 (2010).
Fortier, L. A., Barker, J. U., Strauss, E. J., McCarrel, T. M. & Cole, B. J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 469, 2706–2715 (2011).
US National Library of Medicine. Study of TG-C in patients with grade 3 degenerative joint diseases of the knee. ClinialTrial.gov [online], (2012).
Chen, W., Tabata, Y. & Tong, Y. W. Fabricating tissue engineering scaffolds for simultaneous cell growth and drug delivery. Curr. Pharm. Des. 16, 2388–2394 (2010).
Andreas, K. et al. Biodegradable insulin-loaded PLGA microspheres fabricated by three different emulsification techniques: investigation for cartilage tissue engineering. Acta Biomater. 7, 1485–1495 (2011).
Park, J. S., Yang, H. N., Woo, D. G., Chung, H. M. & Park, K. H. In vitro and in vivo chondrogenesis of rabbit bone marrow-derived stromal cells in fibrin matrix mixed with growth factor loaded in nanoparticles. Tissue Eng. Part A 15, 2163–2175 (2009).
da Silva, M. A., Martins, A., Teixeira, A. A., Reis, R. L. & Neves, N. M. Impact of biological agents and tissue engineering approaches on the treatment of rheumatic diseases. Tissue Eng. Part B Rev. 16, 331–339 (2010).
Ghannam, S., Bouffi, C., Djouad, F., Jorgensen, C. & Noel, D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res. Ther. 1, 2 (2010).
European Medicines Agency, CAT Secretariat & US Food and Drug Administration. Regen. Med. 6, 90–96 (2011).
Halme, D. G. & Kessler, D. A. FDA regulation of stem-cell-based therapies. N. Engl. J. Med. 355, 1730–1735 (2006).
McAllister, T. N., Dusserre, N., Maruszewski, M. & L'Heureux, N. Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regen. Med. 3, 925–937 (2008).
Gerlier, L. et al. The cost utility of autologous chondrocytes implantation using ChondroCelect(R) in symptomatic knee cartilage lesions in Belgium. Pharmacoeconomics 28, 1129–1146 (2010).
Rayment, E. A. & Williams, D. J. Concise review: mind the gap: challenges in characterizing and quantifying cell- and tissue-based therapies for clinical translation. Stem Cells 28, 996–1004 (2010).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (Grant: DFG SI 569/7-1) and the Bundesministerium für Bildung und Forschung (Grant: BCRT 0315848A).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching of data, writing, and reviewing/editing of the manuscript before submission. J. Ringe and M. Sittinger made equal contributions to discussion of content.
Corresponding author
Ethics declarations
Competing interests
M. Sittinger is a shareholder of CellServe GmbH (Berlin, Germany) and BioRetis GmbH (Berlin, Germany), and works as consultant for BioTissue Technologies GmbH (Freiburg, Germany) that develops tissue transplants for the regeneration of bone and cartilage. G.R. Burmester and J. Ringe have no competing interests.
Rights and permissions
About this article
Cite this article
Ringe, J., Burmester, G. & Sittinger, M. Regenerative medicine in rheumatic disease—progress in tissue engineering. Nat Rev Rheumatol 8, 493–498 (2012). https://doi.org/10.1038/nrrheum.2012.98
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.98
This article is cited by
-
Applications of Polypeptide Hydrogels in Cartilage-Regeneration Engineering
Journal of Shanghai Jiaotong University (Science) (2023)
-
Anti-inflammatory effect of hesperidin enhances chondrogenesis of human mesenchymal stem cells for cartilage tissue repair
Journal of Inflammation (2018)
-
Basic science of osteoarthritis
Journal of Experimental Orthopaedics (2016)
-
Repair and tissue engineering techniques for articular cartilage
Nature Reviews Rheumatology (2015)
-
Selecting the right biological scaffold for tissue engineering
Nature Reviews Rheumatology (2014)