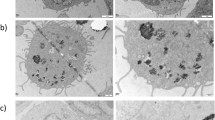
Abstract
Joint replacement surgery is one of the success stories of modern medicine, restoring mobility, diminishing pain and improving the overall quality of life for millions of people. Unfortunately, wear of these prostheses over time generates debris, which activates an innate immune response that can ultimately lead to periprosthetic resorption of bone (osteolysis) and failure of the implant. Over the past decade, the biological interactions between the particulate debris from various implant materials and the immune system have begun to be better understood. The wear debris induces a multifaceted immune response encompassing the generation of reactive oxygen species and damage-associated molecular patterns, Toll-like receptor signaling and NALP3 inflammasome activation. Acting alone or in concert, these events generate chronic inflammation, periprosthetic bone loss and decreased osteointegration that ultimately leads to implant failure.
Key Points
-
Wear debris is generated by the movements of the articulating surfaces of a joint replacement under load
-
Microparticle wear debris induces “frustrated phagocytosis” and multinucleated giant cell fusion
-
Nanoparticle wear debris induces endosomal destabilization and NALP3 inflammasome activation
-
Ultra-high-molecular-weight polyethylene polymeric wear debris and damage-associated molecular patterns induce activation of Toll-like receptors 2 and 4
-
Metal wear debris and metal ions can induce a type IV hypersensitivity reaction
-
The multifaceted myelomonocytic inflammatory response induced by wear debris increases osteoclastogenesis and promotes periprosthetic osteolysis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bozic, K. J. et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J. Bone Joint Surg. Am. 91, 1614–1620 (2009).
Canadian Institute for Health Information. Hip and Knee Replacements in Canada—Canadian Joint Replacement Registry (CJRR) 2008–2009 Annual Report [online] (2009).
Lee, K. & Goodman, S. B. Current state and future of joint replacements in the hip and knee. Expert Rev. Med. Devices 5, 383–393 (2008).
Buckwalter, A. et al. Results of Charnley total hip arthroplasty with use of improved femoral cementing techniques. a concise follow-up, at a minimum of twenty-five years, of a previous report. J. Bone Joint Surg. Am. 88, 1481–1485 (2006).
Sundfeldt, M., Carlsson, L. V., Johansson, C. B., Thomsen, P. & Gretzer, C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 77, 177–97 (2006).
Goodman, S. B. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 28, 5044–5048 (2007).
Harris, W. H. Wear and periprosthetic osteolysis: the problem. Clin. Orthop. Relat. Res. 393, 66–70 (2001).
Looney, R. J., Schwarz, E. M., Boyd, A. & O'Keefe, R. J. Periprosthetic osteolysis: an immunologist's update. Curr. Opin. Rheumatol. 18, 80–7 (2006).
Sargeant, A. & Goswami, T. Pathophysiological aspects of hip implants. J. Surg. Orthop. Adv. 15, 111–112 (2006).
Purdue, P. E., Koulouvaris, P., Potter, H. G., Nestor, B. J. & Sculco, T. P. The cellular and molecular biology of periprosthetic osteolysis. Clin. Orthop. Relat. Res. 454, 251–261 (2007).
Ito, S., Matsumoto, T., Enomoto, H. & Shindo, H. Histological analysis and biological effects of granulation tissue around loosened hip prostheses in the development of osteolysis. J. Orthop. Sci. 9, 478–487 (2004).
Stea, S. et al. Cytokines and osteolysis around total hip prostheses. Cytokine 12, 1575–1579 (2000).
O'Neill, L. A. Immunology. How frustration leads to inflammation. Science 320, 619–620 (2008).
Maitra, R. et al. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol. Immunol. 47, 175–184 (2009).
Hirayama, T. et al. Toll-like receptors and their adaptors are regulated in macrophages after phagocytosis of lipopolysaccharide-coated titanium particles. J. Orthop. Res. 28, 984–992 (2011).
Maitra, R., Clement, C. C., Crisi, G. M., Cobelli, N. & Santambrogio, L. Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PLoS One 3, e2438 (2008).
Islam, A. S., Beidelschies, M. A., Huml, A. & Greenfield, E. M. Titanium particles activate Toll-like receptor 4 independently of lipid rafts in RAW264.7 murine macrophages. J. Orthop. Res. 29, 211–217 (2011).
Piccinini, A. M. & Midwood, K. S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 672395 (2010).
Tamaki, Y. et al. Increased expression of toll-like receptors in aseptic loose periprosthetic tissues and septic synovial membranes around total hip implants. J. Rheumatol. 36, 598–608 (2009).
Holt, G., Murnaghan, C., Reilly, J. & Meek, R. M. The biology of aseptic osteolysis. Clin. Orthop. Relat. Res. 460, 240–252 (2007).
Charnley, J. Arthroplasty of the hip. A new operation. Lancet 1, 1129–1132 (1961).
äkelä, K . et al. Cemented versus cementless total hip replacements in patients fifty-five years of age or olderwith rheumatoid arthritis. J. Bone Joint Surg. Am. 93, 178–186 (2011).
Thomas, G. E. et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J. Bone Joint Surg. Am. 93, 716–722 (2011).
Huo, M. H. & Brown, B. S. What's new in hip arthroplasty. J. Bone Joint Surg. Am. 85-A, 1852–1864 (2003).
Baudriller, H., Chabrand, P. & Moukoko, D. Modeling UHMWPE wear debris generation. J. Biomed. Mater. Res. B Appl. Biomater. 80, 479–485 (2007).
Ingham, E. & Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 26, 1271–1286 (2005).
Endo, M. et al. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc. Inst. Mech. Eng. H 216, 111–122 (2002).
Atienza, C. Jr & Maloney, W. J. Highly cross-linked polyethylene bearing surfaces in total hip arthroplasty. J. Surg. Orthop. Adv. 17, 27–33 (2008).
Doorn, P. F. et al. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. 42, 103–111 (1998).
Yoon, T. R., Rowe, S. M., Jung, S. T., Seon, K. J. & Maloney, W. J. Osteolysis in association with a total hip arthroplasty with ceramic bearing surfaces. J. Bone Joint Surg. Am. 80, 1459–1468 (1998).
Hatton, A. et al. Alumina–alumina artificial hip joints. Part I: a histological analysis and characterisation of wear debris by laser capture microdissection of tissues retrieved at revision. Biomaterials 23, 3429–3440 (2002).
Tipper, J. L. et al. Alumina-alumina artificial hip joints. Part II: characterisation of the wear debris from in vitro hip joint simulations. Biomaterials 23, 3441–3448 (2002).
Elfick, A., Green, S., Krikler, S. & Unsworth, A. The nature and dissemination of UHMWPE wear debris retrieved from periprosthetic tissue of THR. J. Biomed. Mater. Res. A 65, 95–108 (2003).
Schmalzried, T., Dorey, F. & McKellop, H. The multifactorial nature of polyethylene wear in vivo. J. Bone Joint Surg. Am. 80, 1234–1242 (1998).
Galvin, A. L. et al. Wear and biological activity of highly crosslinked polyethylene in the hip under low serum protein concentrations. Proc. Inst. Mech. Eng. H 221, 1–10 (2007).
Schmalzried, T., Peters, P., Maurer, B., Bragdon, C. & Harris, W. Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfaces. J. Arthroplasty 11, 322–331 (1996).
Goldsmith, A. A., Dowson, D., Isaac, G. H. & Lancaster, J. G. A comparative joint simulator study of the wear of metal-on-metal and alternative material combinations in hip replacements. Proc. Inst. Mech. Eng. H 214, 39–47 (2000).
Dorlot, J., Christel, P. & Meunier, A. Wear analysis of retrieved alumina heads and sockets of hip prostheses. J. Biomed. Mater. Res. 23 (A3 Suppl.), 299–310 (1989).
Boehler, M. et al. Long-term results of uncemented alumina acetabular implants. J. Bone Joint Surg. Br. 76, 53–59 (1994).
Kuzyk, P. et al. Cross-linked versus conventional polyethylene for total hip replacement: a meta-analysis of randomised controlled trials. J. Bone Joint Surg. Br. 93, 593–600 (2011).
Urban, R. M. et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Joint Surg. Am. 82, 457–476 (2000).
Delaunay, C., Petit, I., Learmonth, I. D., Oger, P. & Vendittoli, P. A. Metal-on-metal bearings total hip arthroplasty: the cobalt and chromium ions release concern. Orthop. Traumatol. Surg. Res. 96, 894–904 (2010).
Keegan, G. M., Learmonth, I. D. & Case, C. P. Orthopaedic metals and their potential toxicity in the arthroplasty patient: a review of current knowledge and future strategies. J. Bone Joint Surg. Br. 89, 567–573 (2007).
Malviya, A., Ramaskandhan, J., Holland, J. & Lingard, E. Metal-on-metal total hip arthroplasty. J. Bone Joint Surg. Am. 92, 1675–1683 (2010).
Charnley, J. Fracture of femoral prostheses in total hip replacement. A clinical study. Clin. Orthop. Relat. Res. 111, 105–120 (1975).
Harris, W. H., Schiller, A. L., Scholler, J. M., Freiberg, R. A. & Scott, R. Extensive localized bone resorption in the femur following total hip replacement. J. Bone Joint Surg. Am. 58, 612–618 (1976).
Ollivere, B., Darrah, C., Barker, T., Nolan, J. & Porteous, M. J. Early clinical failure of the Birmingham metal-on-metal hip resurfacing is associated with metallosis and soft-tissue necrosis. J. Bone Joint Surg. Br. 91, 1025–1030 (2009).
Kwon, Y. M. et al. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J. Bone Joint Surg. Br. 92, 356–361 (2010).
Pandit, H. et al. Pseudotumours associated with metal-on-metal hip resurfacings. J. Bone Joint Surg. Br. 90, 847–851 (2008).
Pandit, H. et al. Necrotic granulomatous pseudotumours in bilateral resurfacing hip arthoplasties: evidence for a type IV immune response. Virchows Arch. 453, 529–534 (2008).
Davies, A. P., Willert, H. G., Campbell, P. A., Learmonth, I. D. & Case, C. P. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J. Bone Joint Surg. Am. 87, 18–27 (2005).
Huber, M., Reinisch, G., Zenz, P., Zweymüller, K. & Lintner, F. Postmortem study of femoral osteolysis associated with metal-on-metal articulation in total hip replacement: an analysis of nine cases. J. Bone Joint Surg. Am. 92, 1720–1731 (2010).
Willert, H. G. et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J. Bone Joint Surg. Am. 87, 28–36 (2005).
Brach del Prever, E. M. et al. The biological reactivity of polyethylene wear debris is related with sterilisation methods of UHMWPE. Chir. Organi. Mov. 88, 291–304 (2003).
Bosetti, M., Zanardi, L., Bracco, P., Costa, L. & Cannas, M. In vitro evaluation of the inflammatory activity of ultra-high molecular weight polyethylene. Biomaterials 24, 1419–1426 (2003).
Renò, F., Bracco, P., Costa, L. & Cannas, M. Cytotoxicity and MMP-9 activation induced in human mononuclear cells by UHMWPE oxidation. Biomaterials 23, 3645–3650 (2002).
Smith, R. A., Maghsoodpour, A. & Hallab, N. J. In vivo response to cross-linked polyethylene and polycarbonate-urethane particles. J. Biomed. Mater. Res. A 93, 227–234 (2010).
Goodman, S. B. & Ma, T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials 31, 5045–5050 (2010).
Lenz, R. et al. Response of human osteoblasts exposed to wear particles generated at the interface of total hip stems and bone cement. J. Biomed. Mater. Res. A 89, 370–378 (2009).
Kanaji, A. et al. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone 45, 528–533 (2009).
Wang, C. T., Lin, Y. T., Chiang, B. L., Lee, S. S. & Hou, S. M. Over-expression of receptor activator of nuclear factor-kappaB ligand (RANKL), inflammatory cytokines, and chemokines in periprosthetic osteolysis of loosened total hip arthroplasty. Biomaterials 31, 77–82 (2010).
Haynes, D. R. et al. The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis. J. Bone Joint Surg. Br. 83, 902–911 (2001).
Teitelbaum, S. L. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 170, 427–435 (2007).
Wu, Y., Humphrey, M. B. & Nakamura, M. C. Osteoclasts—the innate immune cells of the bone. Autoimmunity 41, 183–194 (2008).
Boyce, B. F., Schwarz, E. M. & Xing, L. Osteoclast precursors: cytokine-stimulated immunomodulators of inflammatory bone disease. Curr. Opin. Rheumatol. 18, 427–432 (2006).
Kahn, A. J., Teitelbaum, S. L., Malone, J. D. & Krukowski, M. The relationship of monocytic cells to the differentiation and resorption of bone. Prog. Clin. Biol. Res. 110 Pt B, 239–248 (1982).
Alnaeeli, M. & Teng, Y. T. Dendritic cells differentiate into osteoclasts in bone marrow microenvironment in vivo. Blood 113, 264–265 (2009).
Maitra, R. et al. Dendritic cell-mediated in vivo bone resorption. J. Immunol. 185, 1485–1491 (2010).
Lamkanfi, M. & Dixit, V. M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 227, 95–105 (2009).
Caicedo, M. S. et al. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J. Orthop. Res. 27, 847–854 (2009).
St Pierre, C. A. et al. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J. Orthop. Res. 28, 1418–1424 (2010).
Cassel, S. L. et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl Acad. Sci. USA 105, 9035–9040 (2008).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–647 (2008).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Freeman, T. A., Parvizi, J., Della Valle, C. J. & Steinbeck, M. J. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis Tissue Repair 2, 5 (2009).
Hallab, N., Merritt, K. & Jacobs, J. J. Metal sensitivity in patients with orthopaedic implants. J. Bone Joint Surg. Am. 83-A, 428–436 (2001).
Campbell, P. et al. in Histopathology of Metal-on-Metal Hip Joint Tissues (eds Rieker, C. et al.) 167–180 (World Tribology Forum in Arthroplasty, Gottingen, 2000).
Campbell, P. et al. The John Charnley Award: a study of implant failure in metal-on-metal surface arthroplasties. Clin. Orthop. Relat. Res. 453, 35–46 (2006).
Gamerdinger, K. et al. A new type of metal recognition by human T cells: contact residues for peptide-independent bridging of T cell receptor and major histocompatibility complex by nickel. J. Exp. Med. 197, 1345–1353 (2003).
Budinger, L. & Hertl, M. Immunologic mechanisms in hypersensitivity reactions to metal ions: an overview. Allergy 55, 108–115 (2000).
Nasorri, F. et al. Activation of nickel-specific CD4+ T lymphocytes in the absence of professional antigen-presenting cells. J. Invest. Dermatol. 118, 172–179 (2002).
Thierse, H. J., Gamerdinger, K., Junkes, C., Guerreiro, N. & Weltzien, H. U. T cell receptor (TCR) interaction with haptens: metal ions as non-classical haptens. Toxicology 209, 101–107 (2005).
Chiu, R., Ma, T., Smith, R. L. & Goodman, S. B. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J. Biomed. Mater. Res. A 89, 242–247 (2009).
Nawrocki, B., Polette, M., Burlet, H., Birembaut, P. & Adnet, J. J. Expression of gelatinase A and its activator MT1-MMP in the inflammatory periprosthetic response to polyethylene. J. Bone Miner. Res. 14, 288–294 (1999).
Takei, I. et al. Messenger ribonucleic acid expression of 16 matrix metalloproteinases in bone–implant interface tissues of loose artificial hip joints. J. Biomed. Mater. Res. 52, 613–620 (2000).
Nam, J. L. et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann. Rheum. Dis. 69, 976–986 (2010).
Rémy, A., Avouac, J., Gossec, L. & Combe, B. Clinical relevance of switching to a second tumour necrosis factor-alpha inhibitor after discontinuation of a first tumour necrosis factor-alpha inhibitor in rheumatoid arthritis: a systematic literature review and meta-analysis. Clin. Exp. Rheumatol. 29, 96–103 (2011).
De Giglio, E. et al. Development and characterization of rhVEGF-loaded poly(HEMA-MOEP) coatings electrosynthesized on titanium to enhance bone mineralization and angiogenesis. Acta Biomater. 6, 282–290 (2010).
Zhang, Z. Y. et al. A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 31, 8684–8695 (2010).
Hildebrandt, C., Büth, H. & Thielecke, H. A scaffold-free in vitro model for osteogenesis of human mesenchymal stem cells. Tissue Cell 43, 91–100 (2011).
Author information
Authors and Affiliations
Contributions
N. Cobelli, B. Scharf, G. M. Crisi and L. Santambrogio researched data for the article. G. M. Crisi and J. Hardin made substantial contributions to the discussion of content. N. Cobelli, B. Scharf and L. Santambrogio wrote the article. N. Cobelli and L. Santambrogio performed review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cobelli, N., Scharf, B., Crisi, G. et al. Mediators of the inflammatory response to joint replacement devices. Nat Rev Rheumatol 7, 600–608 (2011). https://doi.org/10.1038/nrrheum.2011.128
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.128
This article is cited by
-
Current application of dexamethasone-incorporated drug delivery systems for enhancing bone formation
Journal of Pharmaceutical Investigation (2023)
-
Specific inflammatory osteoclast precursors induced during chronic inflammation give rise to highly active osteoclasts associated with inflammatory bone loss
Bone Research (2022)
-
Inhibitory role of Annexin A1 in pathological bone resorption and therapeutic implications in periprosthetic osteolysis
Nature Communications (2022)
-
Osteoclast fusion and bone loss are restricted by interferon inducible guanylate binding proteins
Nature Communications (2021)
-
The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification
Inflammation (2021)