Abstract
Amyloidosis comprises a group of diseases characterized by the extracellular deposition of insoluble fibrillar proteins. This mechanism generates different clinical syndromes depending on the site and extent of organ involvement. Amyloidosis is classified into categories of systemic and localized disease. Systemic amyloidosis is further subdivided into a hereditary familial form (for example, ATTR amyloidosis), a reactive form (AA amyloidosis), dialysis-related (Aβ2M) amyloidosis and immunoglobulin light chain (AL) amyloidosis. Treatment can be symptomatic, directed at the affected organ, or can be directed at reducing the production of the abnormal proteins with different strategies. Despite advances in treatment, the prognosis is still poor and depends on the underlying disease as well as the type and degree of dysfunction in involved organs. Early diagnosis is essential because patients with advanced disease are generally unable to undergo intensive therapy. Patients with systemic amyloidosis often present to a rheumatologist not only because the disease can include musculoskeletal and articular symptoms but also because it can be associated with chronic rheumatic diseases. This Review discusses the clinical features of amyloidosis and its rheumatic manifestations. The various types of amyloidosis, as well their prognosis and treatment, are also presented.
Key Points
-
Amyloidosis describes a heterogeneous group of diseases in which normally soluble plasma proteins are deposited in the extracellular space in an abnormal, insoluble, fibrillar form
-
Rheumatic diseases constitute the most frequent cause of AA amyloidosis in Western countries, and AL amyloidosis should be considered in patients with proteinuria, cardiomyopathy, hepatomegaly, neuropathy, gastrointestinal and musculoskeletal symptoms
-
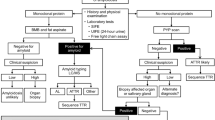
Diagnosis of amyloidosis requires a multidisciplinary approach, including clinical examination, biochemical tests, imaging and genetic analysis, and should be confirmed in a tissue sample by use of Congo red staining in polarized light
-
Fine-needle aspiration of abdominal fat is an easy, noninvasive, safe, fast, and inexpensive technique that demonstrates amyloid deposits in approximately 80–88% of patients
-
Early diagnosis and unequivocal typing of the amyloid deposits are crucial for prognosis and therapy, and age of onset, the type of amyloidosis and cardiac involvement are the main negative prognostic factors
-
Current therapies center on reducing the supply of the amyloid precursor protein to decrease new amyloid formation and perhaps facilitate regression of existing deposits
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sipe, J. D. & Cohen, A. S. History of the amyloid fibril. J. Struct. Biol. 130, 88–98 (2000).
Kelly, J. W. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr. Opin. Struct. Biol. 8, 101–106 (1998).
Quintas, A., Vaz, D. C., Cardoso, I., Saraiva M. J. & Brito, R. M. Comparative calorimetric study of non-amyloidogenic and amyloidogenic variants of the homotetrameric protein transthyretin. Biophys. Chem. 15, 61–67 (2000).
Reixach, N., Deechongkit, S., Jiang, X., Kelly, J. W. & Buxbaum, J. N. Tissue damage in the amyloidoses: transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc. Natl Acad. Sci. USA 101, 2817–2822 (2004).
Dubrey, S. W., Cha, K., Skinner, M., LaValley, M. & Falk, R. H. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart 78, 74–82 (1997).
Liao, R. et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 104, 1594–1597 (2001).
Obici, L., Perfetti, V., Palladini, G., Moratti, R. & Merlini, G. Clinical aspects of systemic amyloid diseases. Biochem. Biophys. Acta 1753, 11–22 (2005).
Haas, M., Meehan, S. M., Karrison, T. G, & Spargo, B. H. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am. J. Kidney Dis. 30, 621–631 (1997).
Kushwaha, S. S., Fallon, J. T. & Fuster, V. Restrictive cardiomyopathy. N. Engl. J. Med. 336, 267–276 (1997).
Kyle, R. A. Amyloidosis. Circulation 91, 1269–1271 (1995).
Park, M. A. et al. Primary (AL) hepatic amyloidosis: clinical features and natural history in 98 patients. Medicine (Baltimore) 8 2, 291–298 (2003).
Nestle, F. O. & Burg, G. Bilateral carpal tunnel syndrome as a clue for the diagnosis of systemic amyloidosis. Dermatology 202, 353–355 (2001).
Prokaeva, T. et al. Soft tissue, joint, and bone manifestation of AL amyloidosis: clinical presentation, molecular features and survival. Arthritis Rheum. 56, 3858–3868 (2007).
Pras, M., Itzchaki, M., Prelli, F., Dollberg, L. & Frangine, B. Amyloid arthropathy: characterization of the amyloid protein. Clin. Exp. Rheumatol. 3, 327–331 (1985).
Liepnieks, J. J., Burt, C. & Benson, M. D. Shoulder-pad sign of amyloidosis: structure of an Ig κ III protein. Scand. J. Immunol. 54, 404–408 (2001).
Merlini, G. Primary (AL) amyloidosis. Amyloid 8, 54–55 (2001).
Gertz, M. A., Kyle, R. A., Griffin, W. L. & Hunder, G. G. Jaw claudication in primary systemic amyloidosis. Medicine (Baltimore) 65, 173–179 (1986).
Salvarani, C. et al. Primary systemic amyloidosis presenting as giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 37, 1621–1626 (1994).
Churchill, C. H., Abril, A., Krishna, M., Callman, M. L. & Ginsburg, W. W. Jaw claudication in primary amyloidosis: unusual presentation of a rare disease. J. Rheumatol. 30, 2283–2286 (2003).
Stebbing, J., Buetens, O., Hellman, D. & Stone, J. Secondary amyloidosis associated with giant cell arteritis/polymyalgia rheumatica. J. Rheumatol. 26, 2698–2700 (1999).
Altiparmark, M. R. et al. Giant cell arteritis and secondary amyloidosis: the natural history. Scand. J. Rheumatol. 30, 114–116 (2001).
Jardinet, D., Westhovens, R. & Peeters, J. Sicca syndrome as an initial symptom of amyloidosis. Clin. Rheumatol. 17, 546–548 (1998).
Gester, J. G., Landry, M. & Dudler, J. Scleroderma-like changes of the hands in primary amyloidosis. J. Rheumatol. 27, 2275–2277 (2000).
Mandl, L. A., Folkerth, R. D., Pick, M. A., Weinblatt, M. E. & Gravallese, E. M. Amyloid myopathy masquerading as polymyositis. J. Rheumatol. 27, 949–952 (2000).
Spuler, S., Emslie-Smith, A. & Engel, A. G. Amyloid myopathy: an underdiagnosed entity. Ann. Neurol. 43, 719–728 (1998).
Gertz, M. A. & Kyle, R. A. Myopathy in primary systemic amyloidosis. J. Neurol. Neurosurg. Psychiatry 60, 655–660 (1996).
Doriguzzi, C., Mongini, T., Troni, W. & Monga, G. Early sarcolemmal dysfunction in skeletal muscle amyloidosis. J. Neurol. 234, 52–54 (1987).
Chapin, J. E., Kornfeld, M. & Harris, A. Amyloid myopathy: characteristic features of a still underdiagnosed disease. Muscle Nerve 31, 266–272 (2005).
Choufani, E. B. et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood 97, 1885–1887 (2001).
Myllykangas-Luosujärvi, R., Aho, K., Kautiainen, H. & Hakala, M. Amyloidosis in a nationwide series of 1666 subjects with rheumatoid arthritis who died during 1989 in Finland. Rheumatology (Oxford) 38, 499–503 (1999).
Laasko, M., Mutru, O., Isomäki, H. & Koota, K. Mortality from amyloidosis and renal diseases in patients with rheumatoid arthritis. Ann. Rheum. Dis. 45, 663–667 (1986).
Strobel, E. S. & Fritschka, E. Renal diseases in ankylosing spondylitis: review of the literature illustrated by case reports. Clin. Rheumatol. 17, 524–530 (1998).
Immonen, K. et al. No improvement in survival of patients with amyloidosis associated with inflammatory rheumatic diseases—data from the Finnish national registry for kidney diseases. J. Rheumatol. 35, 1334–1338 (2008).
Lehtinen, K. The mortality and causes of death of patients with “hypergamma type” of ankylosing spondylitis. Scand. J. Rheumatol. 12, 3–4 (1983).
Sokka, T., Möttönen, T. & Hannonen, P. Mortality in early “sawtooth” treated rheumatoid arthritis patients during the first 8–14 years. Scand. J. Rheumatol. 28, 282–287 (1999).
Laiho, K., Tiitinen, S., Kaarela, K., Helin, H. & Isomäki, H. Secondary amyloidosis has decreased in patients with inflammatory joint diseases in Finland. Clin. Rheumatol. 18, 122–123 (1999).
Hazenberg, B. P. C. & Van Rijswijk, M. H. Where has secondary amyloid gone? Ann. Rheum. Dis. 59, 577–579 (2000).
Suzuki, A. et al. Cause of death in 81 autopsied patients with rheumatoid arthritis. J. Rheumatol. 21, 33–36 (1994).
Wiland, P., Wojatala, R., Goodacre, J. & Szechinki, J. The prevalence of subclinical amyloidosis in Polish patients with rheumatoid arthritis. Clin. Rheumatol. 23, 193–198 (2004).
Levine, R. A. Amyloid disease of the liver: Correlation of clinical, functional, and morphological features in forty-seven patients. Am. J. Med. 33, 349–357 (1962).
Hawkins, P. N. Serum amyloid P component scintigraphy for diagnosis and monitoring amyloidosis. Curr. Opin. Nephrol. Hypertens. 11, 649–655 (2002).
Brandt, K., Cathcart, E. S. & Cohen, A. S. A clinical analysis of the course and prognosis of forty-two patients with amyloidosis. Am. J. Med. 44, 955–969 (1968).
Gertz, M. A. & Kyle, R. A. Secondary systemic amyloidosis: response and survival in 64 patients. Medicine (Baltimore) 70, 246–256 (1991).
Tanaka, F. et al. Clinical outcome and survival of secondary (AA) amyloidosis. Clin. Exp. Rheumatol. 21, 343–346 (2003).
Joss, N., McLaughlin, K. Simpson, K. & Boulton-Jones, J. M. Presentation, survival and prognostic markers in AA amyloidosis. Q. J. Med. 93, 535–542 (2000).
Lachmann, H. J. et al. Natural history and outcome in systemic AA amyloidosis. N. Engl. J. Med. 356, 2361–2371 (2007).
Bergesio, F. et al. Renal involvement in systemic amyloidosis: an Italian collaborative study on survival and renal outcome. Nephrol. Dial. Transplant. 2, 941–951 (2008).
Çakar, N. et al. Familial Mediterranean fever (FMF)-associated amyloidosis in childhood: clinical features, course and outcome. Clin. Exp. Rheumatol. 19 (Suppl. 24), S63–S67 (2001).
Kallinich, T. et al. Two familial cases with tumor necrosis factor receptor-associated periodic syndrome caused by a non-cysteine mutation (T50M) in the TNFRSF1A gene associated with severe multiorganic amyloidosis. J. Rheumatol. 31, 2519–2522 (2004).
Schwarz, R. E., Dralle, H., Linke, R. P., Nathrath, W. B. & Neumann, K. H. Amyloid goitre and arthritides after kidney transplantation in a patient with systemic amyloidosis and Muckle–Wells syndrome. Am. J. Clin. Pathol. 92, 821–825 (1989).
Hoffman, H. M., Wanderer, A. A. & Broide, D. H. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J. Allergy Clin. Immunol. 108, 615–620 (2001).
Obici, L. et al. First report of systemic reactive (AA) amyloidosis in a patient with the hyperimmunoglobulinemia D with periodic fever syndrome. Arthritis Rheum. 50, 2966–2969 (2004).
Gejyo, F. & Narita, I. Current clinical and pathogenetic understanding of β2m- amyloidosis in long-term haemodialysis patients. Nephrology 8 (Suppl. 2), S45–S49 (2003).
Drueke, T. B. Dialysis-related amyloidosis. Nephrol. Dial. Transplant. 1, 58–64 (1998).
Di Raimondo, C. R. Casey, T. T., Di Raimondo, C. V. & Stone, W. J. Pathologic fractures associated with idiopathic amyloidosis of bone in chronic hemodialysis patients. Nephron 43, 22–27 (1986).
Noel, L. H. et al. Tissue distribution of dialysis amyloidosis. Clin. Nephrol. 27, 175–178 (1987).
Gal, R., Korzets, A., Schwartz, A., Rath-Wolfson, L. & Gafter, U. Systemic distribution of beta 2-microglobulin-derived amyloidosis in patients who undergo long-term hemodialysis. Report of seven cases and review of the literature. Arch. Pathol. Lab. Med. 118, 718–721 (1994).
Gorevic, P. D. et al. β-2 microglobulin is an amyloidogenic protein in man. J. Clin. Invest. 76, 2425–2429 (1985).
Sethi, D. & Gower, P. E. Synovial-fluid beta-2-microglobulin levels in dialysis arthropathy. N. Engl. J. Med. 315, 1419–1420 (1986).
Moe, S. M. & Chen, N. X. The role of the synovium and cartilage in the pathogenesis of beta (2)-microglobulin amyloidosis. Semin. Dial. 14, 127–130 (2001).
Giorgetti, S. et al. Beta 2-microglobulin isoforms display a heterogeneous affinity for type I collagen. Protein Sci. 14, 696–702 (2005).
Westermark, P., Bergstrom, J., Solomon, A., Murphy, C. & Sletten, K. Transthyretin-derived senile systemic amyloidosis: clinicopathologic and structural considerations. Amyloid 10, 48–54 (2003).
Breedveld, F. C., Markusse, H. M. & MacFarlane, J. D. Subcutaneous fat biopsy in the diagnosis of amyloidosis secondary to chronic arthritis. Clin. Exp. Rheumatol. 7, 407–410 (1989).
Klemi, P. J., Sorsa, S. & Happonen, R. P. Fine-needle aspiration biopsy from subcutaneous fat. An easy way to diagnose secondary amyloidosis. Scand. J. Rheumatol. 16, 429–431 (1987).
Duston, M. A., Skinner, M., Meenan, R. F. & Cohen, A. S. Sensitivity, specificity, and predictive value of abdominal fat aspiration for the diagnosis of amyloidosis. Arthritis Rheum. 32, 82–85 (1989).
Libbey, C. A., Skinner, M. & Cohen, A. S. Use of abdominal fat tissue aspirate in the diagnosis of systemic amyloidosis. Arch. Intern. Med. 143, 1549–1552 (1983).
Van Gameren, I. I., Hazenberg, B. P. C., Bijzet, J. & van Rijswijk, M. H. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 54, 2015–2021 (2006).
Orfila, C. et al. Unsuitable value of abdominal fat tissue aspirate examination for the diagnosis of amyloidosis in long-term hemodialysis patients. Am. J. Nephrol. 8, 454–456 (1988).
Merlini, G. et al. β2-Microglobulin does not deposit in abdominal fat tissue of long-term hemodialysis patients. In Amyloid and Amyloidosis 1990: Proc. of the VIth International Symp. on Amyloidosis, held in Oslo, Norway, 5–8 August 1990 (Eds Natvig, J. et al.) 801–804 (Springer, 1991).
Tishler, M., Pras, M. & Yaron, M. Abdominal fat tissue aspirate in amyloidosis of familial Mediterranean fever. Clin. Exp. Rheumatol. 6, 395–397 (1988).
Livneh, A. & Langevitz, P. Diagnostic and treatment concerns in familial Mediterranean fever. Baillieres Best Pract. Res. Clin. Rheumatol. 14, 477–498 (2000).
Hazenberg, B. P. et al. Diagnostic performance of amyloid A protein quantification in fat tissue of patients with clinical AA amyloidosis. Amyloid 14, 133–140 (2007).
Giorgadze, T., Baloch, Z. W., Thaler, E. R. & Gupta, P. K. Unsuspected systemic amyloidosis diagnosed by fine-needle aspiration of the salivary gland: case report. Diagn. Cytopathol. 31, 57–59 (2004).
Hachulla, E. et al. Labial salivary gland biopsy is a reliable test for the diagnosis of primary and secondary amyloidosis. Arthritis Rheum. 36, 691–697 (1993).
Gafni, J. & Sohar, E. Rectal biopsy for the diagnosis of amyloidosis. Am. J. Med. Sci. 24 0, 332–336 (1960).
Kyle, R. A., Spencer, R. J. & Dahlin, D. C. Value of rectal biopsy in the diagnosis of primary systemic amyloidosis. Am. J. Med. Sci. 251, 501–506 (1966).
Saraiva, M. J. M. Sporadic cases of hereditary systemic amyloidosis. N. Engl. J. Med. 346, 1818–1819 (2002).
Kyle, R. A. et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 346, 564–569 (2002).
Lachmann, H. J. et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N. Engl. J. Med. 346, 1786–1791 (2002).
Comenzo, R. L., Zhou, P., Fleisher, M., Clark, B. & Teruya-Feldstein, J. Seeking confidence in the diagnosis of systemic AL (Ig light chain) amyloidosis: patients can have both monoclonal gammopathies and hereditary amyloid proteins. Blood 107, 3489–3491 (2006).
Rocken, C., Schwotzer, E. B., Linke, R. P. & Saeger, W. The classification of amyloid deposits in clinicopathological practice. Histopathology 29, 325–335 (1996).
Arbustini, E. et al. Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid 9, 108–114 (2002).
Lavatelli, F. et al. Amyloidogenic and associated proteins in systemic amyloidosis proteome of adipose tissue. Mol. Cell. Proteomics 7, 1570–1583 (2008).
Vrana, J. A. et al. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114, 4957–4959 (2009).
Hawkins, P. N., Lavender, J. P. & Pepys, M. B. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N. Engl. J. Med. 323, 508–513 (1990).
Jager, P. L. et al. Kinetic studies with Iodine-123-labeled serum amyloid P component in patients with systemic AA and AL amyloidosis and assessment of clinical value. J. Nucl. Med. 39, 699–706 (1998).
Falk, R. H., Comenzo, R. L. & Skinner, M. The systemic amyloidoses. N. Engl. J. Med. 337, 898–909 (1997).
Kyle, R. A. & Gertz, M. A. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin. Hematol. 32, 45–59 (1995).
Gillmore, J. D., Lovat, L. B., Persey, M. R., Pepys, M. B. & Hawkins, P. N. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet 358, 24–29 (2001).
Merlini, G. & Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 349, 583–596 (2003).
Comenzo, R. L. et al. Dose-intensive melphalan with blood stem-cell support for the treatment of AL (amyloid light-chain) amyloidosis: survival and responses in 25 patients. Blood 91, 3662–3670 (1998).
Comenzo, R. L. & Gertz, M. A. Autologous stem cell transplantation for primary systemic amyloidosis. Blood 99, 4276–4282 (2002).
Skinner, M. et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann. Intern. Med. 140, 85–93 (2004).
Sanchorawala, V. et al. High-dose intravenous melphalan and autologous stem cell transplantation as initial therapy or following two cycles of oral chemotherapy for the treatment of AL amyloidosis: results of a prospective randomized trial. Bone Marrow Transplant. 33, 381–388 (2004).
Gertz, M. A., Lacy, M. Q. & Dispenzieri, A. Myeloablative chemotherapy with stem cell rescue for the treatment of primary systemic amyloidosis: a status report. Bone Marrow Transplant. 25, 465–470 (2000).
Bradstock, K., Clancy, R., Uther, J., Basten, A. & Richards, J. The successful treatment of primary amyloidosis with intermittent chemotherapy. Aust. NZ J. Med. 8, 176–179 (1978).
Corkery, J., Bern, M. M. & Tullis, J. L. Resolution of amyloidosis and plasma-cell dyscrasia with combination chemotherapy. Lancet 2, 425–426 (1978).
Kyle, R. A. et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone and colchicine. N. Engl. J. Med. 336, 1202–1207 (1997).
Palladini, G. et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis ineligible for stem cell transplantation. Blood 1 03, 2936–2938 (2004).
Palladini, G. et al. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL). Blood 105, 2949–2951 (2005).
Sanchorawala, V. et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood 109, 492–496 (2007).
Reece, D. et al. Weekly and twice-weekly bortezomib in patients with systemic AL-amyloidosis: results of a phase 1 dose-escalation study. Blood 11 4, 1489–1497 (2009).
Wechalekar, A. D., Lachmann, H. J., Offer, M., Hawkins, P. N. & Gillmore, J. D. Efficacy of bortezomib in systemic AL amyloidosis with relapsed/refractory clonal disease. Haematologica 93, 295–298 (2008).
Kastritis, E. et al. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica 92, 1351–1358 (2007).
Gottenberg, J.-E. et al. Anti-tumor necrosis factor α therapy in fifteen patients with AA amyloidosis secondary to inflammatory arthritides: a followup report of tolerability and toxicity. Arthritis Rheum. 48, 2019–2024 (2003).
Drewe, E., Huggins, M. L., Morgan, A. G., Cassidy, M. J. & Powell, R. J. Treatment of renal amyloidosis with etanercept in tumour necrosis factor receptor-associated periodic syndrome. Rheumatology (Oxford) 43, 1405–1408 (2004).
Amital, H. & Ben-Chetrit, E. Therapeutic approaches to familial Mediterranean fever. What do we know and where are we going to? Clin. Exp. Rheumatol. 22 (Suppl. 34), S4–S7 (2004).
Duzova, A. et al. Role of A-SAA in monitoring subclinical inflammation and in colchicine dosage in familial Mediterranean fever. Clin. Exp. Rheumatol. 21, 509–514 (2003).
Leslie, K. S. et al. Phenotype, genotype, and sustained response to anakinra in 22 patients with autoinflammatory disease associated with CIAS-1/NALP3 mutations. Arch. Dermatol. 142, 1591–1597 (2006).
Kisilevsky, R. et al. Arresting amyloidosis in vivo using small-molecule anionic sulphonates or sulphates: implications for Alzheimer's disease. Nat. Med. 1, 143–148 (1995).
Gervais, F., Chalifour, R., Kong, X., Morissette, C. & Paquette, J. GAG mimetics: advance in multiple therapeutic areas. Amyloid and Amyloidosis: the Proc. of the IXth International Symp. on Amyloidosis, Budapest, July 15–21, 2001 (Eds Bely, M. & Apathy, A.) 589–592 (2001).
Dember, L. et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N. Engl. J. Med. 356, 2349–2360 (2007).
Winchester, J. F., Salsberg, J. A. & Levin, N. W. Beta-2 microglobulin in ESRD: an in-depth review. Adv. Renal Replace. Ther. 10, 279–309 (2003).
Yamamoto, S. & Gejyo, F. Historical background and clinical treatment of dialysis-related amyloidosis. Biochem. Biophys. Acta 1753, 4–10 (2005).
Kelly, J. W. et al. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv. Protein Chem. 50, 161–181 (1997).
Cardoso, I. et al. Transthyretin fibrillogenesis entails the assembly of monomers: a molecular model for in vitro assembled transthyretin amyloid-like fibrils. J. Mol. Biol. 317, 683–695 (2002).
Kelly, J. W. Alternative conformations of amyloidogenic proteins govern their behavior. Curr. Opin. Struct. Biol. 6, 11–17 (1996).
Hammarstrom, P., Wiseman, R. L., Powers, E. T. & Kelly, J. W. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science 299, 713–716 (2003).
Miller, S. R., Sekijima, Y. & Kelly, J. W. Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab. Invest. 84, 545–552 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perfetto, F., Moggi-Pignone, A., Livi, R. et al. Systemic amyloidosis: a challenge for the rheumatologist. Nat Rev Rheumatol 6, 417–429 (2010). https://doi.org/10.1038/nrrheum.2010.84
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.84
This article is cited by
-
Kidney disease in a child with familial Mediterranean fever: Answers
Pediatric Nephrology (2022)
-
Monoclonal gammopathy of renal significance (MGRS)-related AL amyloidosis complicated by amyloid myopathy: a case report
BMC Nephrology (2021)
-
How to manage primary amyloidosis
Leukemia (2012)
-
Renal transplantation in patients with familial Mediterranean fever
Clinical Rheumatology (2012)
-
Light chain (AL) amyloidosis: update on diagnosis and management
Journal of Hematology & Oncology (2011)