Abstract
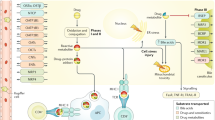
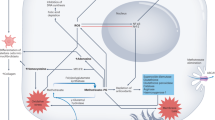
Antirheumatic agents are among commonly used drugs associated with adverse hepatic reactions. Sulfasalazine and azathioprine are among the most important causes of acute hepatotoxicity. Because such a large number of people take NSAIDs, even the rare occurrence of hepatotoxicity from these agents might contribute substantially to the total burden of drug-induced liver disease. A wide spectrum of hepatotoxic effects is described with antirheumatic drugs. Studies investigating genetic susceptibility to diclofenac hepatotoxicity have expanded our understanding of the potential drug-specific, class-specific and general factors involved in its pathogenesis, and methotrexate-associated liver disease demonstrates the interaction between drug, host and environmental factors that determines the likelihood and magnitude of liver disease. Infliximab therapy is associated with typical drug-induced autoimmune hepatitis. Although validated causality assessment methods have been used to objectively assess the strength of the association between a drug and a clinical event, in practice the diagnosis of drug-induced liver injury (DILI) involves a clinical index of suspicion, pattern recognition, the establishment of a temporal relationship between drug exposure and the adverse event, and the exclusion of alternative explanations for the clinical presentation. Detailed understanding of genetic and environmental factors underlying an individual's susceptibility would enable risk reduction and potentially primary prevention of hepatotoxicity.
Key Points
-
Antirheumatic agents are among commonly used drugs associated with hepatotoxic effects ranging from acute drug-induced liver injury (DILI) to chronic drug-associated liver disease and drug-induced autoimmune hepatitis
-
Diclofenac hepatotoxicity demonstrates that the pathogenesis of acute 'idiosyncratic' DILI is a multistep process involving interaction between metabolic and immunologic factors
-
Drug regimen and genetic susceptibility contribute to the pathogenesis of methotrexate-associated chronic liver disease; however, environmental factors are the major determinants of the extent of liver injury
-
Avoiding the use of a particular drug in those with recognized risk factors (e.g. methotrexate in those with diabetes, obesity or excessive alcohol consumption) whenever clinically feasible, could reduce the risk of serious DILI
-
Novel serum markers and imaging techniques can detect evidence of liver fibrosis non-invasively and their use in the monitoring of methotrexate-associated chronic liver disease should be evaluated
-
The strong association between a common HLA haplotype and lumiracoxib-induced liver injury raises the possibility of pre-prescription testing and primary prevention of acute idiosyncratic DILI
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Olson, H. et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 32, 56–67 (2000).
Laine, L. et al. How common is diclofenac-associated liver injury? Analysis of 17,289 arthritis patients in a long-term prospective clinical trial. Am. J. Gastroenterol. 104, 356–362 (2009).
Traversa, G. et al. Cohort study of hepatotoxicity associated with nimesulide and other non-steroidal anti-inflammatory drugs. BMJ 327, 18–22 (2003).
Scottish Government Health Directorates. Lumiracoxib: suspension of UK licences with immediate effect (non urgent) cascade within 48 hours. SGHD on SHOW [online], (2007).
de Abajo, F. J., Montero, D., Madurga, M. & García Rodríguez, L. A. Acute and clinically relevant drug-induced liver injury: a population based case–control study. Br. J. Clin. Pharmacol. 58, 71–80 (2004).
Paulose-Ram, R. et al. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III). Pharmacoepidemiol. Drug Saf. 12, 315–326 (2003).
Aithal, G. P. & Day, C. P. Nonsteroidal anti-inflammatory drug-induced hepatotoxicity. Clin. Liver Dis. 11, 563–575 (2007).
Rubenstein, J. H. & Laine, L. Systematic review: the hepatotoxicity of non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 20, 373–380 (2004).
Perez Gutthann, S. & García Rodríguez, L. A. The increased risk of hospitalizations for acute liver injury in a population with exposure to multiple drugs. Epidemiology 4, 496–501 (1993).
García Rodríguez, L. A., Williams, R., Derby, L. E., Dean, A. D. & Jick, H. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch. Intern. Med. 154, 311–316 (1994).
Banks, A. T., Zimmerman, H. J., Ishak, K. G. & Harter, J. G. Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology 22, 820–827 (1995).
Chitturi, S. & George, J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin. Liver Dis. 22, 169–183 (2002).
Aithal, G. P. Diclofenac-induced liver injury: a paradigm of idiosyncratic drug toxicity. Expert Opin. Drug Saf. 3, 519–523 (2004).
Aithal, G. P., Day, C. P., Leathart, J. B. & Daly, A. K. Relationship of polymorphism in CYP2C9 to genetic susceptibility to diclofenac-induced hepatitis. Pharmacogenetics 10, 511–518 (2000).
Thibaudeau, J. et al. Characterization of common UGT1A8, UGT1A9, and UGT2B7 variants with different capacities to inactivate mutagenic 4-hydroxylated metabolites of estradiol and estrone. Cancer Res. 66, 125–133 (2006).
Duguay, Y., Báár, C., Skorpen, F. & Guillemette, C. A novel functional polymorphism in the uridine diphosphate–glucuronosyltransferase 2B7 promoter with significant impact on promoter activity. Clin. Pharmacol. Ther. 75, 223–233 (2004).
Daly, A. K. et al. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology 132, 272–281 (2007).
Jensen, L. E. et al. A common ABCC2 promoter polymorphism is not a determinant of the risk of spina bifida. Birth Defects Res. A Clin. Mol. Teratol. 70, 396–399 (2004).
Haenisch, S. et al. Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J. 7, 56–65 (2007).
Dai, D. et al. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 11, 597–607 (2001).
Bahadur, N. et al. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6a-hydroxylase activity in human liver microsomes. Biochem. Pharmacol. 64, 1579–1589 (2002).
Niemi, M. et al. Polymorphism in CYP2C8 is associated with reduced plasma concentrations of repaglinide. Clin. Pharmacol. Ther. 74, 380–387 (2003).
Aithal, G. P. et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology 39, 1430–1440 (2004).
Pirmohamed, M., Naisbitt, D. J., Gordon, F. & Park, B. K. The danger hypothesis—potential role in idiosyncratic drug reactions. Toxicology 181–182, 55–63 (2002).
Kaplowitz, N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 4, 489–499 (2005).
Tarazi, E. M., Harter, J. G., Zimmerman, H. J., Ishak, K. G. & Eaton, R. A. Sulindac-associated hepatic injury: analysis of 91 cases reported to the Food and Drug Administration. Gastroenterology 104, 569–574 (1993).
Bolder, U. et al. Sulindac is excreted into bile by a canalicular bile salt pump and undergoes a cholehepatic circulation in rats. Gastroenterology 117, 962–971 (1999).
Jobanputra, P. et al. Hepatotoxicity associated with sulfasalazine in inflammatory arthritis: a case series from a local surveillance of serious adverse events. BMC Musculoskelet. Disord. 9, 48 (2008).
Bocquet, H., Bagot, M. & Roujeau, J. C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin. Cutan. Med. Surg. 15, 250–257 (1996).
Ohtani, T., Hiroi, A., Sakurane, M. & Furukawa, F. Slow acetylator genotypes as a possible risk factor for infectious mononucleosis-like syndrome induced by salazosulfapyridine. Br. J. Dermatol. 148, 1035–1039 (2003).
Kitas, G. D., Farr, M., Waterhouse, L. & Bacon, P. A. Influence of acetylator status on sulphasalazine efficacy and toxicity in patients with rheumatoid arthritis. Scand. J. Rheumatol. 21, 220–225 (1992).
Emery, P. et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 39, 655–665 (2000).
US Food and Drug Administration. Arthritis Advisory Committee slides March 5, 2003: Arava®(leflunomide). CDER 2003 meeting documents [online], (2003).
Matteson, E. & Cush, J. J. Reports of leflunomide hepatotoxicity in patients with rheumatoid arthritis. August 2001. ACR Hotline [online], (2001).
Alcorn, N. Saunders, S. & Madhok, R. Benefit–risk assessment of leflunomide: an appraisal of leflunomide in rheumatoid arthritis 10 years after licensing. Drug Saf. 32, 1123–1134 (2009).
Helliwell, P. S. & Taylor, W. J. Treatment of psoriatic arthritis and rheumatoid arthritis with disease modifying drugs—comparison of drugs and adverse reactions. J. Rheumatol. 35, 472–476 (2008).
Basset, C., Vadrot, J., Denis, J., Poupon, J. & Zafrani, E. S. Prolonged cholestasis and ductopenia following gold salt therapy. Liver Int. 23, 89–93 (2003).
Fleischner, G. M. et al. Light- and electron-microscopical study of a case of gold salt-induced hepatotoxicity. Hepatology 14, 422–425 (1991).
Watkins, P. B., Schade, R., Mills, A. S., Carithers, R. L. Jr & van Thiel, D. H. Fatal hepatic necrosis associated with parenteral gold therapy. Dig. Dis. Sci. 33, 1025–1029 (1988).
Gisbert, J. P. et al. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm. Bowel Dis. 13, 1106–1114 (2007).
Jeurissen, M. E. Boerbooms, A. M., van de Putte, L. B. & Kruijsen, M. W. Azathioprine induced fever, chills, rash, and hepatotoxicity in rheumatoid arthritis. Ann. Rheum. Dis. 49, 25–27 (1990).
Desmet, V. J. Vanishing bile duct syndrome in drug-induced liver disease. J. Hepatol. 26 (Suppl. 1), 31–35 (1997).
Dubinsky, M. C. et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology 122, 904–915 (2002).
Sparrow, M. P., Hande, S. A., Friedman, S., Cao, D. & Hanauer, S. B. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin. Gastroenterol. Hepatol. 5, 209–214 (2007).
Ansari, A. et al. Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment. Pharmacol. Ther. 28, 734–741 (2008).
Chandran, V., Schentag, C. T. & Gladman, D. D. Reappraisal of the effectiveness of methotrexate in psoriatic arthritis: results from a longitudinal observational cohort. J. Rheumatol. 35, 469–471 (2008).
Taylor, W. J. et al. Drug use and toxicity in psoriatic disease: focus on methotrexate. J. Rheumatol. 35, 1454–1457 (2008).
Hassan, W. Methotrexate and liver toxicity: role of surveillance liver biopsy. Conflict between guidelines for rheumatologists and dermatologists. Ann. Rheum. Dis. 55, 273–275 (1996).
Kocharla, L. et al. Monitoring methotrexate toxicity in juvenile idiopathic arthritis. J. Rheumatol. 36, 2813–2818 (2009).
Salliot, C. & van der Heijde, D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann. Rheum. Dis. 68, 1100–1104 (2009).
Aithal, G. P. Dangerous liaisons: drug, host and the environment. J. Hepatol. 46, 995–998 (2007).
Kremer, J. M. Toward a better understanding of methotrexate. Arthritis Rheum. 50, 1370–1382 (2004).
Desouza, C., Keebler, M., McNamara, D. B. & Fonseca, V. Drugs affecting homocysteine metabolism: impact on cardiovascular risk. Drugs 62, 605–616 (2002).
Mato, J. M. & Lu, S. C. Homocysteine, the bad thiol. Hepatology 41, 976–979 (2005).
Basseri, S. & Austin, R. C. ER stress and lipogenesis: a slippery slope toward hepatic steatosis. Dev. Cell 15, 795–796 (2008).
Curtis, J. R. et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann. Rheum. Dis. 69, 43–47 (2010).
Aithal, G. P. et al. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: are serial liver biopsies justified? Aliment. Pharmacol. Ther. 19, 391–399 (2004).
Fisher, M. C. & Cronstein, B. N. Metaanalysis of methylenetetrahydrofolate reductase (MTHFR) polymorphisms affecting methotrexate toxicity. J. Rheumatol. 36, 539–545 (2009).
Bohanec Grabar, P., Logar, D., Lestan, B. & Dolzan, V. Genetic determinants of methotrexate toxicity in rheumatoid arthritis patients: a study of polymorphisms affecting methotrexate transport and folate metabolism. Eur. J. Clin. Pharmacol. 64, 1057–1068 (2008).
Kooloos, W. M. et al. Functional polymorphisms and methotrexate treatment outcome in recent-onset rheumatoid arthritis. Pharmacogenomics 11, 163–175 (2010).
Ranganathan, P. et al. Methotrexate (MTX) pathway gene polymorphisms and their effects on MTX toxicity in Caucasian and African American patients with rheumatoid arthritis. J. Rheumatol. 35, 572–579 (2008).
Miele, L. et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J. Hepatol. 51, 778–786 (2009).
Whiting-O'Keefe, Q. E., Fye, K. H. & Sack, K. D. Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am. J. Med. 90, 711–716 (1991).
Rosenberg, P. et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J. Hepatol. 46, 1111–1118 (2007).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Ji, C. & Kaplowitz, N. ER stress: can the liver cope? J. Hepatol. 45, 321–333 (2006).
Katchamart, W., Trudeau, J., Phumethum, V. & Bombardier, C. Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No.: CD008495. doi:10.1002/14651858.CD008495 (2010).
Reshamwala, P. A., Kleiner, D. E. & Heller, T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology 44, 7–14 (2006).
Abraham, S., Begum, S. & Isenberg, D. Hepatic manifestations of autoimmune rheumatic diseases. Ann. Rheum. Dis. 63, 123–129 (2004).
Breen, D. P., Marinaki, A. M., Arenas, M. & Hayes, P. C. Pharmacogenetic association with adverse drug reactions to azathioprine immunosuppressive therapy following liver transplantation. Liver Transpl. 11, 826–833 (2005).
Seiderer, J. et al. Nodular regenerative hyperplasia: a reversible entity associated with azathioprine therapy. Eur. J. Gastroenterol. Hepatol. 18, 553–555 (2006).
Sokolove, J. et al. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann. Rheum. Dis. 69, 1612–1617 (2010).
Strand, V., Kimberly, R. & Isaacs, J. D. Biologic therapies in rheumatology: lessons learned, future directions. Nat. Rev. Drug Discov. 6, 75–92 (2007).
US Food and Drug Administration. Important drug warning. FDA MedWatch [online], (2004).
Suissa, S., Ernst, P., Hudson, M., Bitton, A. & Kezouh, A. Newer disease-modifying antirheumatic drugs and the risk of serious hepatic adverse events in patients with rheumatoid arthritis. Am. J. Med. 117, 87–92 (2004).
Tobon, G. J. Cañas, C., Jaller, J. J., Restrepo, J. C. & Anaya, J. M. Serious liver disease induced by infliximab. Clin. Rheumatol. 26, 578–581 (2007).
Mancini, S., Amorotti, E., Vecchio, S., Ponz de Leon, M. & Roncucci, L. Infliximab-related hepatitis: discussion of a case and review of the literature. Intern. Emerg. Med. 193–200 (2010).
Kong, Y. C., Wei, W. Z. & Tomer, Y. Opportunistic autoimmune disorders: from immunotherapy to immune dysregulation. Ann. NY Acad. Sci. 1183, 222–236 (2010).
Danan, G. & Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J. Clin. Epidemiol. 46, 1323–1330 (1993).
Goldkind, L. & Laine, L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: lessons learned from the bromfenac experience. Pharmacoepidemiol. Drug Saf. 15, 213–220 (2006).
Björnsson, E. & Olsson, R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 42, 481–489 (2005).
British Society for Rheumatology. National guidelines for the monitoring of second line drugs. Produced by the British Society for Rheumatology July 2000. Guidelines archive [online], (2000).
Senior, J. R. Monitoring for hepatotoxicity: what is the predictive value of liver “function” tests? Clin. Pharmacol. Ther. 85, 331–334 (2009).
Thomas, J. A. & Aithal, G. P. Monitoring liver function during methotrexate therapy for psoriasis: are routine biopsies really necessary? Am. J. Clin. Dermatol. 6, 357–363 (2005).
Smith, C. H. & Barker, J. N. Psoriasis and its management. BMJ 333, 380–384 (2006).
Chalmers, R. J. et al. Replacement of routine liver biopsy by procollagen III aminopeptide for monitoring patients with psoriasis receiving long-term methotrexate: a multicentre audit and health economic analysis. Br. J. Dermatol. 152, 444–450 (2005).
MacDonald, A. & Burden, A. D. Noninvasive monitoring for methotrexate hepatotoxicity. Br. J. Dermatol. 152, 405–408 (2005).
Bossuyt, P. M. et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for reporting of diagnostic accuracy. Clin. Chem. 49, 1–6 (2003).
Guha, I. N. et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 47, 455–460 (2008).
Laharie, D. et al. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case–control study. J. Hepatol. 53, 1035–1040 (2010).
Abdelmalek, M. F. et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology 50, 1818–1826 (2009).
Singer, J. B. et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat. Genet. 42, 711–714 (2010).
Aithal, G. P. & Daly, A. K. Preempting and preventing drug-induced liver injury. Nat. Genet. 42, 650–651 (2010).
Acknowledgements
The author is grateful to Dr. Charles Paulding, Novartis Institutes for BioMedical Research and Dr. Muhammad F. Dawwas, Nottingham University Hospitals, for allowing him to discuss their unpublished data in this Review. The author also thanks Dr. Philip Kaye, Nottingham University Hospitals NHS Trust, for providing histology slides used in this article. C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Aithal, G. Hepatotoxicity related to antirheumatic drugs. Nat Rev Rheumatol 7, 139–150 (2011). https://doi.org/10.1038/nrrheum.2010.214
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.214
This article is cited by
-
Study design for development of novel safety biomarkers of drug-induced liver injury by the translational safety biomarker pipeline (TransBioLine) consortium: a study protocol for a nested case–control study
Diagnostic and Prognostic Research (2023)
-
Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases
Stem Cell Research & Therapy (2022)
-
Effectiveness of new selenium-enriched mutated probiotics in reducing inflammatory effects of piroxicam medication in liver and kidney
Inflammopharmacology (2022)
-
Treatment of rheumatoid arthritis with conventional, targeted and biological disease-modifying antirheumatic drugs in the setting of liver injury and non-alcoholic fatty liver disease
Rheumatology International (2022)
-
Safety Profile of Upadacitinib up to 3 Years in Psoriatic Arthritis: An Integrated Analysis of Two Pivotal Phase 3 Trials
Rheumatology and Therapy (2022)