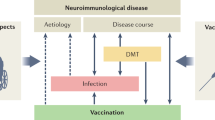
Abstract
Bacterial and viral infections have been shown to induce relapses and accelerate the progression of multiple sclerosis (MS). Vaccination to prevent communicable disease in such patients is, therefore, of key importance. Reports of potentially detrimental effects of immunization on the course of MS, however, have prompted patients and physicians to adopt a cautious attitude towards the use of vaccines. The risks associated with a number of vaccines have been investigated in patients with MS. Vaccines against some diseases, such as tetanus and hepatitis B, are not associated with an elevated risk of MS exacerbation, whereas vaccines against other diseases, such as yellow fever, are contraindicated in patients with MS. Many patients with MS receive immunosuppressive or immunomodulatory therapy, which could make them more susceptible to infectious diseases and might also affect their ability to respond to immunization. Here, we review the indications for and possible adverse effects of vaccines in patients with MS, and address issues of vaccination in the context of immunomodulatory therapy for MS.
Key Points
-
Infections can trigger a relapse in patients with multiple sclerosis (MS), warranting appropriate vaccination
-
Concerns of potential adverse reactions after immunization in MS have led to restriction of the use of some vaccines
-
Some vaccines—in particular, inactivated vaccines—are safe and beneficial in MS
-
Immunosuppressive and immunomodulatory treatment for MS could affect vaccine efficacy
Similar content being viewed by others
Introduction
Vaccination is a mainstay in the prevention of communicable infectious diseases. This intervention is particularly important in patients with chronic debilitating diseases, who are prone to infections that could aggravate their symptoms.1,2
Numerous studies in patients with multiple sclerosis (MS) have shown that the risk of relapse is increased by infections. For example, shortly after a bacterial urinary tract infection, a viral respiratory infection or gastroenteritis, patients with MS experienced significantly raised relapse rates and enhanced lesion activity as measured with MRI.3 Another study found that infection increased the risk of relapse approximately twofold.4 Moreover, relapses associated with an infection seem to be more prone to causing neurological dysfunction (as measured by the Expanded Disability Status Scale score) than are relapses not associated with infection.4
Vaccination elicits an immune response against modified antigens of the infective agent, which increases the speed and efficacy of the immune response to subsequent exposure to the pathogen, thereby providing protection. However, some evidence suggests that vaccines might inadvertently activate immune responses to autoantigens in patients with autoimmune disease which, in the case of MS, could trigger a relapse. Suggested mechanisms for this process include molecular mimicry (shared epitopes between microorganisms or vaccine antigens and CNS proteins in MS) and polyclonal bystander activation of T lymphocytes (Box 1).5
Concerns have been raised that in addition to eliciting relapses, vaccination might also trigger the onset of MS in susceptible individuals.5 However, a recent extensive review from the US Institute of Medicine did not find sufficient evidence to accept or reject a causal relationship between the onset of MS and vaccination against measles, mumps and rubella (MMR), influenza, hepatitis A, hepatitis B, human papilloma virus (HPV), diphtheria, tetanus, acellular pertussis, or meningococcal disease.6 Furthermore, pooled analyses found no evidence that vaccination against tuberculosis with the Bacillus Calmette–Guérin (BCG) preparation, or against hepatitis B, influenza, measles, typhoid fever, diphtheria or tetanus, was associated with an increased risk of developing MS.7
These conclusions are based on extensive studies applying principles laid down by the WHO.8 These principles require a number of criteria to be fulfilled before a causal link can be considered between a distinct vaccine and subsequent emergence of adverse events—in this instance, exacerbation or worsening of MS (Box 2).
Another important issue related to the use of vaccines in patients with MS is that the expanding repertoire of powerful immunomodulatory and immunosuppressive MS drugs could limit the effectiveness of prophylactic vaccination. Furthermore, on rare occasions some of the new MS drugs are associated with viral infections as serious adverse events, suggesting the need to consider vaccination before initiation of the immunomodulatory or immunosuppressive therapy.
Here, we review studies on the use of vaccines in patients with MS, and identify and discuss risks and benefits of vaccines in patients undergoing treatment with disease-modifying drugs (DMDs).
Standard vaccines
The administration of a vaccine should be considered only when the vaccine-preventable disease has more serious consequences than the potential adverse effects associated with the vaccination.3,9 Patients with MS should be offered immunizations against infections that they are at risk of contracting.10 Existing patient care guidelines such as the UK National Health Service Clinical Guidelines for MS,11 the MS Council for Clinical Practice Guidelines in the US12 or the German Vaccine Commission guidelines13 recommend applying the national standard vaccination strategies to patients with MS. Similar to the vaccination recommendations for healthy adults, those for patients with MS include immunizations against diphtheria, tetanus, influenza and pneumococcus (Table 1). The indications and contraindications in patients with MS are largely the same as for healthy individuals,14 although data specific to MS are lacking for some vaccinations.
Tetanus
The highly immunogenic tetanus vaccine is primarily administered in combination with vaccines against other infectious diseases such as diphtheria, poliomyelitis or pertussis. A tetanus booster vaccination is recommended every 10 years in adults. In a case–control study involving 332 patients with MS and 722 healthy controls, the risk of developing MS or of relapse was not found to be enhanced by prior immunization (OR 0.6; 95% CI 0.4–0.8).15 According to an investigation in 623 patients with MS who were registered in a European MS database, the risk of relapse was not increased after tetanus vaccination (relative risk [RR] 0.75; 95% CI 0.23–2.46).16 Furthermore, in patients receiving combined vaccines, the risk of relapse was lower than in non-vaccinated patients (RR 0.26; 95% CI 0.06–1.12). Similarly, in a comparatively small trial in the Netherlands, no MS exacerbations occurred during the 6 weeks following tetanus vaccination.17 A meta-analysis of case–control studies published between 1966 and 2005, which included 963 patients with MS and 3,126 controls, supports the findings outlined above, including a reduced risk of relapse in patients with MS who have received a tetanus vaccination, compared with those who have not (OR 0.67; 95% CI 0.55–0.81).18 If available, combined vaccines (for example, tetanus, diphtheria, poliomyelitis and pertussis, discussed below) should be used in patients with MS when tetanus vaccination is indicated.
Diphtheria
The diphtheria vaccine is generally administered in combination with the tetanus vaccine. Large-scale studies on the association of diphtheria immunization with the risk of MS exacerbation have not been conducted, but an analysis of patients registered in the European MS database did not show an increased risk of relapse: in patients immunized with a combined tetanus–diphtheria vaccine, the relative risk of relapse was 0.22 (95% CI 0.05–0.99) compared with patients receiving tetanus vaccine alone.16
Influenza
Infection with influenza does not seem to lead to worsening of symptoms in primary progressive MS, according to a study involving 53 patients with this form of the disease.19 By contrast, in 180 patients with relapsing–remitting MS (RRMS), 12 of the 36 patients (33%) who became infected with influenza developed an acute relapse within 6 weeks. Moreover, only four of the 80 patients (5%) who received vaccination against influenza had a relapse.19 In another study, vaccination with standard inactivated influenza vaccines induced comparable T cell responses and influenza A-specific antibody titers to those in healthy controls.20
Vaccination against influenza does not seem to exacerbate MS, although in a small study involving six patients with MS, contrast-enhanced cerebral MRI showed increased lesion activity 15 and 45 days after vaccination in one patient.21 Another study in 11 patients with RRMS, however, did not report clinical exacerbation after vaccination. Cerebral MRI revealed a mean of three new gadolinium-enhancing lesions in the 3-week prevaccination period, compared with only one new inflammatory lesion in the 3-week postvaccination period.22 In an older study, published in 1976, only one of 93 patients with MS showed emerging neurological symptoms after influenza vaccination.23 In addition, a placebo-controlled, double-blind study involving 104 patients with MS did not demonstrate significant differences in the rate of MS exacerbations (3.7% versus 6.1%).24
In June 2009, the WHO announced a pandemic caused by the new H1N1 influenza virus (nH1N1, also known as swine flu), and vaccines against this novel type of influenza A were developed. In a recently published case series of 60 patients with MS, the monovalent nH1N1 vaccine did not increase relapse rates 30, 60, or 90 days following vaccination, compared with equivalent time periods before vaccination.25 In another recent study involving 49 patients with MS who had been vaccinated with the pandemic H1N1 vaccine, the seasonal influenza vaccine or both, none of the patients reported a deterioration of neurological status after vaccination.26 Furthermore, population-based studies have not found an association between vaccination and the triggering of MS relapses.27,28,29 However, in a small case series of 18 patients with MS, relapses occurred within 3 weeks in six of seven patients receiving H1N1 vaccine alone, in four of eight patients receiving simultaneous H1N1 and seasonal vaccine, and in two of three patients receiving seasonal vaccine alone.30
In summary, although further monitoring for adverse effects is warranted when using influenza vaccines, the data obtained so far encourage the use of such vaccines in patients with MS.
Supplementary vaccines
Supplementary vaccines are recommended for adults exposed to situations with an elevated risk of a severe course of infection or worsening of an underlying disease. These situations include chronic disease, professional exposure, travel to endemic areas, and asplenia (Table 2). Of note, trials have not been conducted to investigate the tolerability of vaccines against hepatitis A—the most widely used travel vaccine—or Japanese B encephalitis in MS. Other vaccines of importance in MS are discussed in this section.
Viral diseases
Tick-borne encephalitis
During the past 30 years, rising numbers of tick-borne encephalitis (TBE) cases have been registered across Europe and Russia,31 as the disease spreads from Europe through parts of China to eastern Japan.32 Immunization against this viral disease seems to be warranted in endemic regions, particularly in people who are frequently involved in outdoor activities.
In a small Austrian case–control study, 15 patients with MS from TBE-endemic areas were vaccinated with standard TBE vaccine doses and compared with 15 matched, non-immunized control patients with MS.33 MS progression did not differ between the two groups over 36 months of follow-up. In addition, no differences were observed in the MRI lesion activity and load 45 days after immunization.
Yellow fever
For several countries, yellow fever is the only disease against which international travelers must be vaccinated before they can gain entry to the country. Owing to the risk of severe adverse effects, the yellow fever vaccine is largely contraindicated in immunosuppressed patients, including patients with MS who are undergoing immunomodulatory treatment. Recent studies, however, have shown that immunization is feasible in asymptomatic patients with HIV who have CD4+ helper T-cell counts above 200 per 1 μl.34 A small study in MS showed a significant increase in exacerbation rates within 3 months following yellow fever vaccination compared with prevaccination exacerbation rates.35 In light of this evidence, yellow fever vaccines are not recommended in patients with MS.
Hepatitis B
Following the implementation of a hepatitis B vaccination program in France, CNS demyelination was linked with the vaccine,36 initially leading to governmental compensation of patients with MS.37 However, none of the subsequent studies identified hepatitis B vaccination as a risk factor for developing MS.38,39
Anecdotal observations describing a deterioration of existing CNS demyelinating disease following hepatitis B vaccination40,41 prompted caution in prescribing this vaccine for patients with RRMS, owing to the concern of eliciting an MS relapse. However, in a case-crossover study involving 643 patients with MS who experienced a relapse, hepatitis B vaccination was not found to be a risk factor for developing a relapse during the 2-month risk period following vaccination (RR 0.67; 95% CI 0.20–2.17).16 A study of a French cohort of children aged 0.5–16 years who had experienced a first episode of CNS inflammatory demyelination found that hepatitis B vaccine exposure was not associated with a significant increase in the risk of relapse during a mean follow-up of 5.8 years. The adjusted hazard ratio of developing a second episode of CNS inflammation within 3 years after vaccination was 0.78 (95% CI 0.32–1.89).42 Overall, the hepatitis B vaccination can be considered safe, with no increased risk of developing MS after vaccination36 and no increased risk of relapse in patients with MS.7,16
Human papilloma viruses
Two vaccines—one against two HPV subtypes (HPV-16 and HPV-18) and the other against four HPV subtypes (HPV-6, HPV-11, HPV-16 and HPV-18) that are most commonly associated with cervical cancer and genital warts—are licensed in the USA, Europe and many other countries. Recommendations generally include HPV vaccination for female adolescents aged 11 years and older. Five cases of MS relapses in association with quadrivalent HPV vaccination have been reported,43 and a small case series documented three young females who developed neuromyelitis optica following HPV immunization.44 However, the relapses or development of neuromyelitis optica may well have occurred by chance in this target population. Current data on the effects of the HPV vaccine on established MS are insufficient to determine whether a link exists between vaccination and the triggering of MS relapses.
Measles, mumps and rubella
Immunization against MMR with a live attenuated vaccine is generally recommended in childhood or in unprotected adults after contact with infected individuals. Immunosuppressed patients, however, may develop overt vaccination-induced disease.45 Vaccination against measles should, therefore, be avoided in patients with MS who are receiving immunosuppressive therapy. The effect of MMR vaccination on the course of MS has not been studied. Until further evidence is available, alternative strategies such as passive immunization with hyperimmunoglobulin should be used in the rare cases of unprotected adults with MS who have been exposed to the diseases.
Poliomyelitis
Immunization with the inactivated polio vaccine is recommended for all children, and booster vaccinations are advisable when travelling to an area in which the disease is endemic. To eliminate the risk of vaccine-associated paralytic poliomyelitis, use of the live oral polio vaccine is no longer recommended for routine immunization in many countries. A prospective investigation of the formerly used live polio vaccine in 20 patients with MS did not find evidence of worsening of MS symptoms.46 A recent meta-analysis of clinical trials concluded that the risk of developing MS was not increased after vaccination against polio (OR 0.87; 95% CI 0.61–1.25).7 This meta-analysis included seven nonrandomized trials, conducted between 1989 and 2004, in 570 patients with MS and 725 controls from Europe, Israel and India. Further clinical studies evaluating the risk of triggering MS relapse after vaccination against polio have not been conducted.
Rotavirus
Rotavirus causes gastroenteritis, often in epidemic outbreaks, and is mostly transmitted via the fecal–oral route. Two live attenuated vaccines are available for vaccination of infants. Immunosuppressed patients who have household contact with recently vaccinated infants should follow strict personal hygiene because the vaccine virus is shed after vaccination.47 No studies have yet been conducted on the effect of the vaccine on the course of MS.
Rabies
The current rabies vaccine, which is grown on human diploid cells or chicken fibroblasts, is considerably more tolerable than the formerly used rabies vaccine grown on neural tissues.48 No studies concerning rabies vaccine tolerability in MS have been conducted. Given the invariably fatal course of rabies, immediate active immunization in combination with immunoglobulins is imperative for post-exposure prophylaxis (for example, after being bitten by a rabies-suspected animal) in non-immune individuals.
Varicella zoster virus
Varicella zoster virus (VZV) is a neurotropic virus that causes chickenpox and—on reactivation from latent infection—shingles. VZV infection in adults can be a severe and sometimes life-threatening disease. Vaccination of non-immune adults with live attenuated VZV is recommended before the start of an immunosuppressive or powerful immunomodulatory therapy, and is explicitly required in the case of some DMDs, such as fingolimod,49,50 the sphingosine 1-phosphate receptor modulator that was recently approved for the treatment of MS.51 Immunocompromised individuals who have not been vaccinated and are exposed to VZV should receive hyperimmunoglobulin for post-exposure prophylaxis.
In a 1-year study investigating the neurological status of 47 patients with MS who received VZV vaccination, improvement was observed in 14 individuals (29.8%), deterioration was observed in four patients (8.5%), and no change was seen in 29 patients (61.7%).52 Of note, four patients developed mild vaccine-associated chickenpox. Thus, vaccination may lead to adverse effects in MS and should be avoided, especially in the presence of DMDs.
Bacterial diseases
A notable lack of studies exists on the effects of vaccination against bacterial infections on disease course in MS. Immunization against tuberculosis with the attenuated live BCG vaccine is no longer recommended in some low-prevalence regions because of limited efficacy and a comparatively high rate of complications.53 In one case series, 14 patients with RRMS experienced a 57% reduction of active brain lesions on MRI within 6 months of vaccination,54 and a meta-analysis did not reveal any adverse effects on MS relapse rates.7
Other vaccines against bacterial diseases are widely used, including those to prevent meningococcal, pneumococcal or Haemophilus influenzae type b infections, but none of these has been specifically evaluated in patients with MS. Similarly, travel vaccines such as those against typhoid fever or cholera have not been assessed in relation to MS. This gap in our knowledge emphasizes the need to conduct appropriate studies designed to evaluate the safety of vaccines to prevent major bacterial infections in patients with MS.
Effect of MS drugs on vaccine efficacy
MS is treated with DMDs55 that can modulate or suppress the generation of adaptive immune responses and/or the maintenance of immunological memory. These treatments might diminish the effectiveness of vaccines. Conversely, live attenuated vaccines, such as the yellow fever vaccine, may be contraindicated because of the risk of harmful infection or chronic carriage of the attenuated—but actively replicating—pathogen.35
Case studies have shown good efficacy of vaccination in patients receiving DMD treatment,56 but large studies are scarce, especially in MS. Moreover, systematic prospective studies are virtually absent,12 representing a knowledge gap that demands further clinical studies. No studies have so far been conducted into the effects of immunization in patients receiving the synthetic co-polymer glatiramer acetate, intravenous immunoglobulin (IVIg), or the monoclonal antibody natalizumab, which are all used in the treatment of MS. According to FDA prescribing information, the application of IVIg may impair the immunogenicity of live viral vaccines such as MMR or VZV for 6–12 months.57
The available information on interactions between DMDs and vaccination is discussed in this section. The degree of immunosuppression and the response to immunization can vary considerably between the different groups of DMDs that are used in the treatment of MS.
Corticosteroids
Corticosteroids are widely used in MS and have a broad dose-dependent suppressive effect on immune reactions. This effect could lead, on one hand, to an impaired antibody response following immunization with inactivated vaccines and, on the other, to vaccine-induced infection following immunization with live attenuated vaccines. However, the immune response to influenza or pneumococcal vaccines did not seem to be impaired in recipients of organ transplants, some of whom were receiving corticosteroids.58 This response has not been evaluated in MS.
Interferons
Interferons do not seem to impair immune responses in patients with MS, and these drugs have an antiviral effect.59 In a prospective study of 88 patients with MS who were treated with IFN-β1a, and 77 untreated patients with MS, similar proportions (93.0% and 90.9%, respectively) of each group developed protective immune responses after receiving seasonal influenza vaccine.60 Seasonal influenza vaccines can, therefore, be considered safe and effective in patients with MS receiving IFN-β.
Fingolimod
Fingolimod causes redistribution of lymphocytes51 and may interfere with the immune response. Following influenza vaccination, patients with MS who are being treated with fingolimod have been shown to develop antibody responses comparable to those of healthy controls.61,62 As discussed above, VZV vaccines are live vaccines that are contraindicated during treatment with DMDs or during MS progression. VZV antibody status should, therefore, be determined early in the disease course, before initiation of disease-modifying therapy, to allow vaccination against VZV if necessary.49
Rituximab
Rituximab, a chimeric anti-CD20 monoclonal antibody that induces sustained depletion of B cells, is used to treat lymphomas and various autoimmune diseases. Rituximab and the follow-up humanized anti-CD20 monoclonal antibody ocrelizumab improved outcomes in patients with RRMS in phase II trials.63,64 A number of studies have evaluated antibody responses in patients receiving rituximab, although none has examined patients with MS. Patients with lymphoma who were receiving rituximab did not develop protective antibody titers after vaccination against influenza.65,66 In patients with rheumatoid arthritis, rituximab reduced the humoral response to influenza vaccine67,68,69 but did not affect the cell-mediated response.70 By contrast, rituximab treatment in patients with rheumatoid arthritis was associated with an impaired cell-mediated response after administration of a pneumococcal polysaccharide vaccine.71 These results suggest that inactivated vaccines can be administered during rituximab therapy if vaccination before initiation of rituximab is not feasible, as is the case for seasonal influenza vaccines.
Cytostatic treatment
Studies are lacking on the immune response to vaccination and vaccine safety in patients with MS who are receiving cytostatic treatment.72 Patients with systemic lupus erythematosus (SLE) who were being treated with azathioprine showed a trend towards a diminished antibody response after vaccination against influenza.73 By contrast, antibody responses to pneumococcal vaccine were not impaired in patients with SLE who were receiving azathioprine, cyclophosphamide or prednisolone.74 The effectiveness of hepatitis B vaccination in recipients of organ transplants was reduced by immunosuppression with azathioprine or glucocorticosteroids.75 In addition, in patients with inflammatory bowel disease, TBE vaccination was associated with lower IgG titers and reduced immune protection during azathioprine treatment than during a period without cytostatic treatment.76 No studies have been conducted on the effects of mitoxantrone on the response to immunization.
Implications for clinical practice
Given the possible negative effect of MS drugs on vaccine efficacy, patients should be checked for missing vaccinations before initiation of treatment with immunosuppressive agents or a new immunomodulator, such as fingolimod. Provided that the course of MS allows a delay in starting treatment with such agents, vaccination should be performed in advance.77
The timing of vaccination in relation to MS-specific treatments should be planned according to the DMD manufacturers' recommendations. For example, immunization should be delayed for at least 2 weeks following high-dose glucocorticosteroids. Immunization during mitoxantrone or cyclophosphamide treatment should be performed between drug cycles.
To evaluate the success of vaccination in patients treated with immunosuppressants, antibody testing should be performed 4 weeks after administration of the vaccine. If titers fail to increase, revaccination should be considered. With new DMDs such as fingolimod, antibody status against specific communicable diseases, such as VZV, needs to be monitored and appropriate measures taken to ensure adequate protection against the communicable disease.
Conclusions and future directions
Avoidance of infection in patients with MS generally reduces the risk of relapse and deterioration of health status, which improves quality of life and minimizes additional socioeconomic burden. Patients with MS should receive vaccination against tetanus and diphtheria; adequate protection by previous vaccination against or infection with influenza, pneumococci, pertussis and hepatitis B should be ensured. Whereas inactivated vaccines are generally considered safe for use in patients with MS, live attenuated vaccines may provoke MS relapses, as particularly shown for yellow fever, and can cause vaccine-associated infection in patients undergoing immunosuppressive therapy.
For a number of vaccines, adequate evidence is available to exclude these vaccines as a cause for the induction of MS. Some vaccines might, however, trigger relapses in MS, warranting further investigation. In contrast to vaccines against viral infections, the safety and efficacy of vaccines against bacterial diseases in patients with MS have been evaluated to a very limited extent. In future studies, special attention needs to be directed to vaccinations given during treatment with DMDs, to determine their safety and efficacy in this context.
Review criteria
The US National Library of Medicine's PubMed and the ScienceDirect databases were searched for articles published before or during September 2011. Search terms included “multiple sclerosis”, “CNS inflammation”, “vaccination”, “immunization”, “disease progression” and specific vaccines (for example “tetanus vaccine”). Abstracts of retrieved citations were reviewed and prioritized by relevant content. Full articles were obtained and references were checked for additional material when appropriate. Online material was searched to retrieve relevant guidelines and FDA or European Medicines Agency drug information.
References
Ascherio, A. & Munger, K. L. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann. Neurol. 61, 288–299 (2007).
Handel, A. E., Giovannoni, G., Ebers, G. C. & Ramagopalan, S. V. Environmental factors and their timing in adult-onset multiple sclerosis. Nat. Rev. Neurol. 6, 156–166 (2010).
Correale, J., Fiol, M. & Gilmore, W. The risk of relapses in multiple sclerosis during systemic infections. Neurology 67, 652–659 (2006).
Buljevac, D. et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125, 952–960 (2002).
Salemi, S. & D'Amelio, R. Could autoimmunity be induced by vaccination? Int. Rev. Immunol. 29, 247–269 (2010).
Stratton, K. F. et al. (Eds) Adverse Effects of Vaccines: Evidence and Causality (The National Academies Press, Washington, DC, 2011).
Farez, M. F. & Correale, J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J. Neurol. 258, 1197–1206 (2011).
Global Advisory Committee on Vaccine Safety. Causality assessment of adverse events following immunization. Wkly Epidemiol. Rec. 76, 85–89 (2001).
Jelinek, G. A. & Hassed, C. S. Managing multiple sclerosis in primary care: are we forgetting something? Qual. Prim. Care 17, 55–61 (2009).
Meyer-Olson, D. & Witte, T. Prevention of infections in patients with autoimmune diseases. Nat. Rev. Rheumatol. 7, 198–200 (2011).
National Collaborating Centre for Chronic Conditions (UK). Multiple sclerosis: national clinical guideline for diagnosis and management in primary and secondary care. National Institute for Health and Clinical Excellence [online], (2003).
Rutschmann, O. T., McCrory, D. C. & Matchar, D. B. Immunization and MS: a summary of published evidence and recommendations. Neurology 59, 1837–1843 (2002).
STIKO. Recommendation of the Standing Vaccination Commission. Epidemiol. Bull. 30, 279–298 (2010).
Zipp, F. & Wandinger, K. P. Current recommendations for vaccination in multiple sclerosis. Nervenarzt 73, 384 (2002).
DeStefano, F. et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch. Neurol. 60, 504–509 (2003).
Confavreux, C. et al. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N. Engl. J. Med. 344, 319–326 (2001).
De Keyser, J. Safety of tetanus vaccination in relapsing-remitting multiple sclerosis. Infection 26, 319 (1998).
Hernan, M. A., Alonso, A. & Hernandez-Diaz, S. Tetanus vaccination and risk of multiple sclerosis: a systematic review. Neurology 67, 212–215 (2006).
De Keyser, J., Zwanikken, C. & Boon, M. Effects of influenza vaccination and influenza illness on exacerbations in multiple sclerosis. J. Neurol. Sci. 159, 51–53 (1998).
Moriabadi, N. F. et al. Influenza vaccination in MS: absence of T-cell response against white matter proteins. Neurology 56, 938–943 (2001).
Salvetti, M. et al. Clinical and MRI assessment of disease activity in patients with multiple sclerosis after influenza vaccination. J. Neurol. 242, 143–146 (1995).
Michielsens, B., Wilms, G., Marchal, G. & Carton, H. Serial magnetic resonance imaging studies with paramagnetic contrast medium: assessment of disease activity in patients with multiple sclerosis before and after influenza vaccination. Eur. Neurol. 30, 258–259 (1990).
Sibley, W. A., Bamford, C. R. & Laguna, J. F. Influenza vaccination in patients with multiple sclerosis. JAMA 236, 1965–1966 (1976).
Miller, A. E. et al. A multicenter, randomized, double-blind, placebo-controlled trial of influenza immunization in multiple sclerosis. Neurology 48, 312–314 (1997).
Farez, M. F., Ysrraelit, M. C., Fiol, M. & Correale, J. H1N1 vaccination does not increase risk of relapse in multiple sclerosis: a self-controlled case-series study. Mult. Scler. http://dx.doi.org/10.1177/1352458511417253.
Auriel, E., Gadoth, A., Regev, K. & Karni, A. Seasonal and H1N1v influenza vaccines in MS: safety and compliance. J. Neurol. Sci. http://dx.doi.org/10.1016/j.jns.2011.10.013.
Bardage, C. et al. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ 343, d5956 (2011).
Lee, G. M. et al. H1N1 and seasonal influenza vaccine safety in the vaccine safety datalink project. Am. J. Prev. Med. 41, 121–128 (2011).
Williams, S. E. et al. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination. Vaccine 29, 8302–8308 (2011).
McNicholas, N. & Chataway, J. Relapse risk in patients with multiple sclerosis after H1N1 vaccination, with or without seasonal influenza vaccination. J. Neurol. 258, 1545–1547 (2011).
Mansfield, K. L. et al. Tick-borne encephalitis virus—a review of an emerging zoonosis. J. Gen. Virol. 90, 1781–1794 (2009).
Gao, X., Nasci, R. & Liang, G. The neglected arboviral infections in mainland China. PLoS Negl. Trop. Dis. 4, e624 (2010).
Baumhackl, U., Franta, C., Retzl, J., Salomonowitz, E. & Eder, G. A controlled trial of tick-borne encephalitis vaccination in patients with multiple sclerosis. Vaccine 21 (Suppl. 1), S56–S61 (2003).
Veit, O. et al. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin. Infect. Dis. 48, 659–666 (2009).
Farez, M. F. & Correale, J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch. Neurol. 68, 1267–1271 (2011).
Hocine, M. N. et al. Hepatitis B vaccination and first central nervous system demyelinating events: reanalysis of a case-control study using the self-controlled case series method. Vaccine 25, 5938–5943 (2007).
Jefferson, T. & Heijbel, H. Demyelinating disease and hepatitis B vaccination: is there a link? Drug Saf. 24, 249–254 (2001).
Mikaeloff, Y., Caridade, G., Rossier, M., Suissa, S. & Tardieu, M. Hepatitis B vaccination and the risk of childhood-onset multiple sclerosis. Arch. Pediatr. Adolesc. Med. 161, 1176–1182 (2007).
Ascherio, A. et al. Hepatitis B vaccination and the risk of multiple sclerosis. N. Engl. J. Med. 344, 327–332 (2001).
Terney, D. et al. Multiple sclerosis after hepatitis B vaccination in a 16-year-old patient. Chin. Med. J. (Engl.) 119, 77–79 (2006).
Cabrera-Gomez, J. A. et al. A severe episode in a patient with recurrent disseminated acute encephalitis due to vaccination against hepatitis B. For or against vaccination? Rev. Neurol. 34, 358–363 (2002).
Mikaeloff, Y. et al. Hepatitis B vaccine and risk of relapse after a first childhood episode of CNS inflammatory demyelination. Brain 130, 1105–1110 (2007).
Sutton, I., Lahoria, R., Tan, I., Clouston, P. & Barnett, M. CNS demyelination and quadrivalent HPV vaccination. Mult. Scler. 15, 116–119 (2009).
Menge, T. et al. Neuromyelitis optica following human papillomavirus vaccination. Neurology (in press).
Centers for Disease Control and Prevention (CDC). Measles pneumonitis following measles-mumps-rubella vaccination of a patient with HIV infection, 1993. MMWR Morb. Mortal. Wkly Rep. 45, 603–606 (1996).
Sibley, W. A. & Foley, J. M. Infection and immunization in multiple sclerosis. Ann. NY Acad. Sci. 122, 457–466 (1965).
Anderson, E. J. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect. Dis. 8, 642–649 (2008).
Plotkin, S. A. & Wiktor, T. Rabies vaccination. Annu. Rev. Med. 29, 583–591 (1978).
Winkelmann, A., Loebermann, M., Reisinger, E. C., Hartung, H. P. & Zettl, U. K. Fingolimod treatment for multiple sclerosis. What do we do with varicella? Ann. Neurol. 70, 673–674 (2011).
European Medicines Agency. EPAR summary for the public—Gilenya (fingolimod). European Medicines Agency [online], (2011).
Ingwersen, J. et al. Fingolimod in multiple sclerosis: mechanisms of action and clinical efficacy. Clin. Immunol. http://dx.doi.org/10.1016/j.clim.2011.05.005.
Ross, R. T., Nicolle, L. E. & Cheang, M. The varicella zoster virus: a pilot trial of a potential therapeutic agent in multiple sclerosis. J. Clin. Epidemiol. 50, 63–68 (1997).
Rowland, R. & McShane, H. Tuberculosis vaccines in clinical trials. Expert Rev. Vaccines 10, 645–658 (2011).
Ristori, G. et al. Use of Bacille Calmette–Guèrin (BCG) in multiple sclerosis. Neurology 53, 1588–1589 (1999).
Multiple Sclerosis Therapy Consensus Group. Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. J. Neurol. 255, 1449–1463 (2008).
Fomin, I. et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNFα blockers. Ann. Rheum. Dis. 65, 191–194 (2006).
FDA. Prescribing information for Gammagard Liquid. US Food and Drug Administration [online], (2011).
Briggs, W. A. et al. Influenza vaccination in kidney transplant recipients: cellular and humoral immune responses. Ann. Intern. Med. 92, 471–477 (1980).
Koerner, I., Kochs, G., Kalinke, U., Weiss, S. & Staeheli, P. Protective role of beta interferon in host defense against influenza A virus. J. Virol. 81, 2025–2030 (2007).
Schwid, S. R., Decker, M. D., Lopez-Bresnahan, M. & Rebif–Influenza Vaccine Study Investigators. Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. Neurology 65, 1964–1966 (2005).
Mehling, M. et al. Antigen-specific adaptive immune responses in fingolimod-treated multiple sclerosis patients. Ann. Neurol. 69, 408–413 (2011).
Mehling, M. et al. Cellular and humoral influenza vaccine-specific immune responses in patients with multiple sclerosis treated with FTY720 or interferon-beta. Neurology 74, A372 (2010).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
Kappos, L. et al. Ocrelizumab in relapsing–remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 378, 1779–1787 (2011).
Bedognetti, D. et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin's lymphoma patients treated with rituximab-containing regimens. J. Immunol. 186, 6044–6055 (2011).
Yri, O. E. et al. Rituximab blocks protective serological response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 118, 6769–6771 (2011).
Gelinck, L. B. et al. Poor serological responses upon influenza vaccination in patients with rheumatoid arthritis treated with rituximab. Ann. Rheum. Dis. 66, 1402–1403 (2007).
van Assen, S. et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 62, 75–81 (2010).
Oren, S. et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann. Rheum. Dis. 67, 937–941 (2008).
Arad, U. et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine 29, 1643–1648 (2011).
Rehnberg, M. et al. Vaccination response to protein and carbohydrate antigens in patients with rheumatoid arthritis after rituximab treatment. Arthritis Res. Ther. 12, R111 (2010).
Loebermann, M. et al. Immunization in the adult immunocompromised host. Autoimmun. Rev. http://dx.doi.org/10.1016/j.autrev.2011.05.015.
Abu-Shakra, M. et al. Specific antibody response after influenza immunization in systemic lupus erythematosus. J. Rheumatol. 29, 2555–2557 (2002).
Lipnick, R. N. et al. Pneumococcal immunization in patients with systemic lupus erythematosus treated with immunosuppressives. J. Rheumatol. 12, 1118–1121 (1985).
Wagner, D. et al. Hepatitis B vaccination of immunosuppressed heart transplant recipients with the vaccine Hepa Gene 3 containing pre-S1, pre-S2, and S gene products. Clin. Investig. 72, 350–352 (1994).
Wenzl, H., Jahnel, J., Hoegenauer, C., Hinterleitner, T. & Petritsch, W. Inadequately low antibodies against the tick-borne encephalitis virus in immunized patients with inflammatory bowel disease and azathioprine treatment. Gastroenterology 124, 205A (2003).
Riminton, D. S., Hartung, H. P. & Reddel, S. W. Managing the risks of immunosuppression. Curr. Opin. Neurol. 24, 217–223 (2011).
Department of Health. Immunisation against infectious disease—'The Green Book'—2006 updated edition. Department of Health [online], (2006).
Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2011. MMWR Morb. Mortal. Wkly Rep. 60, 1–4 (2011).
World Health Organization. World immunization schedule. World Health Organization [online], (2010).
Merelli, E. & Casoni, F. Prognostic factors in multiple sclerosis: role of intercurrent infections and vaccinations against influenza and hepatitis B. Neurol. Sci. 21, S853–S856 (2000).
Myers, L. W. et al. Swine influenza virus vaccination in patients with multiple sclerosis. J. Infect. Dis. 136 (Suppl. 3), S546–S554 (1977).
Makela, A., Nuorti, J. P. & Peltola, H. Neurologic disorders after measles–mumps–rubella vaccination. Pediatrics 110, 957–963 (2002).
Ahlgren, C., Oden, A., Toren, K. & Andersen, O. Multiple sclerosis incidence in the era of measles–mumps–rubella mass vaccinations. Acta Neurol. Scand. 119, 313–320 (2009).
Joint Committee on Vaccination and Immunisation. JCVI statement on discontinuation of the routine pneumococcal vaccination programme for adults aged 65 years and older. Department of Health [online], (2011).
Gout, O. & Lyon-Caen, O. Current opinion 2004: The relationship between multiple sclerosis and hepatitis B vaccination in adults [French]. Rev. Neurol. (Paris) 160, 1147–1149 (2004).
Hauben, M., Sakaguchi, M., Patadia, V. & Gerrits, C. M. Hepatitis B vaccination and multiple sclerosis: a data mining perspective. Pharmacoepidemiol. Drug Saf. 16, 943–945 (2007).
Zuckerman, J. N. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J. Med. Virol. 78, 169–177 (2006).
Miller, H., Cendrowski, W. & Shapira, K. Multiple sclerosis and vaccination. Br. Med. J. 2, 210–213 (1967).
Fraser, A., Paul, M., Goldberg, E., Acosta, C. J. & Leibovici, L. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine 25, 7848–7857 (2007).
Author information
Authors and Affiliations
Contributions
M. Loebermann, A. Winkelmann, H.-P. Hartung and U. K. Zettl researched data for the article. All authors contributed to discussion of the article content. M. Loebermann and A. Winkelmann made equal contributions to writing the article. All authors contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. Loebermann has received speaker's honoraria or travel expense compensation from Janssen Cilag, MSD Sharp & Dohme, Novartis Vaccines, Intercell and Gilead. A. Winkelmann has received speaker's honoraria and travel expense compensation from Schering, Bayer HealthCare, Octapharma AG and Merck Serono. H.-P. Hartung has received honoraria for consulting and speaking at scientific symposia from Bayer HealthCare, Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva Sanofi. E. C. Reisinger has received speaker's honoraria and travel expense compensation from Sanofi Pasteur MSD, GlaxoSmithKline, Novartis Behring, Roche and Bayer. U. K. Zettl is on the speakers list of Bayer HealthCare, Biogen Idec, Merck Serono and Aventis/Teva. H. Hengel declares no competing interests.
Rights and permissions
About this article
Cite this article
Loebermann, M., Winkelmann, A., Hartung, HP. et al. Vaccination against infection in patients with multiple sclerosis. Nat Rev Neurol 8, 143–151 (2012). https://doi.org/10.1038/nrneurol.2012.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2012.8
This article is cited by
-
Side effects following vaccination in multiple sclerosis: a prospective, multi-centre cohort study
Scientific Reports (2023)
-
Immune responses following COVID-19 infection in multiple sclerosis patients using immunomodulatory therapy
Acta Neurologica Belgica (2023)
-
Effects of disease-modifying therapy on peripheral leukocytes in patients with multiple sclerosis
Journal of Neurology (2021)
-
Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy
Nature Medicine (2021)
-
Vaccine-associated measles in a patient treated with natalizumab: a case report
BMC Infectious Diseases (2020)