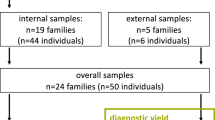
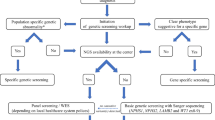
Abstract
Monogenic nephrotic syndrome (nephrotic syndrome caused by a single gene defect) is responsible for only a small percentage of cases of nephrotic syndrome, but information from studies of the unique cohort of patients with this form of the disease has dramatically improved our understanding of the disease pathogenesis. The use of genetic testing in the management of children and adults with nephrotic syndrome poses unique challenges for clinicians in terms of who to test and how to use the information obtained from testing in the clinical setting. In our view, not enough data exist at present to justify the routine genetic testing of all patients with nephrotic syndrome. Testing is warranted, however, in patients with congenital nephrotic syndrome (onset at 0–3 months), infantile nephrotic syndrome (onset at 3–12 months), a family history of nephrotic syndrome, and those in whom nephrotic syndrome is associated with other congenital malformations. The family and/or the patient should be given complete and unbiased information on the potential benefits and risks associated with therapy, including the reported outcomes of treatment in patients with similar mutations. Based on the data available in the literature so far, intensive immunosuppressive treatment is probably not indicated in monogenic nephrotic syndrome if complete or partial remission has not been achieved within 6 weeks of starting treatment. We advocate that family members of individuals with genetic forms of nephrotic syndrome undergo routine genetic testing prior to living-related kidney transplantation. Prospective, multicentre studies are needed to more completely determine the burden of disease caused by monogenic nephrotic syndrome, and randomized controlled trials are needed to clarify the presence or absence of clinical responses of monogenic nephrotic syndrome to available therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
McKinney, P. A., Feltbower, R. G., Brocklebank, J. T. & Fitzpatrick, M. M. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr. Nephrol. 16, 1040–1044 (2001).
[No authors listed] The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J. Pediatr. 98, 561–564 (1981).
Kestila, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell. 1, 575–582 (1998).
Wiggins, R. C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71, 1205–1214 (2007).
Shih, N. Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312–315 (1999).
Dreyer, S. D. et al. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat. Genet. 19, 47–50 (1998).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Zenker, M. et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 13, 2625–2632 (2004).
Kaplan, J. M. et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Boerkoel, C. F. et al. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat. Genet. 30, 215–220 (2002).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Hinkes, B. et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 38, 1397–1405 (2006).
Niaudet, P. & Gubler, M. C. WT1 and glomerular diseases. Pediatr. Nephrol. 21, 1653–1660 (2006).
Berkovic, S. F. et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am. J. Hum. Genet. 82, 673–684 (2008).
Brown, E. J. et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42, 72–76 (2010).
Boyer, O. et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N. Engl. J. Med. 365, 2377–2388 (2011).
Heeringa, S. F. et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 121, 2013–2024 (2011).
Akilesh, S. et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 121, 4127–4137 (2011).
Mele, C. et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 295–306 (2011).
Ozaltin, F. et al. Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 89, 139–147 (2011).
Has, C. et al. Integrin α3 mutations with kidney, lung, and skin disease. N. Engl. J. Med. 366, 1508–1514 (2012).
Büscher, A. K. et al. Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clin. Nephrol. 78, 47–53 (2012).
Ruf, R. G. et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J. Am. Soc. Nephrol. 15, 722–732 (2004).
Hinkes, B. et al. Specific podocin mutations correlate with age of onset in steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol. 19, 365–371 (2008).
Hinkes, B. G. et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119, e907–e919 (2007).
Gbadegesin, R. et al. Mutations in PLCE1 are a major cause of isolated diffuse mesangial sclerosis (IDMS). Nephrol. Dial. Transplant. 23, 1291–1297 (2008).
Gbadegesin, R. A. et al. Inverted formin 2 mutations with variable expression in patients with sporadic and hereditary focal and segmental glomerulosclerosis. Kidney Int. 81, 94–99 (2012).
Boyer, O. et al. Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 239–245 (2011).
Kopp, J. B. et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat. Genet. 40, 1175–1184 (2008).
Genovese, G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Santín, S. et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 6, 1139–1148 (2011).
Conrad, D. F. et al. Variation in genome-wide mutation rates within and between human families. Nat. Genet. 43, 712–714 (2011).
National Center for Biotechnology Information. GeneTests [online], (2012).
Gbadegesin, R. et al. Mutational analysis of NPHS2 and WT1 in frequently relapsing and steroid-dependent nephrotic syndrome. Pediatr. Nephrol. 22, 509–513 (2007).
Büscher, A. K. et al. Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 5, 2075–2084 (2010).
Kitamura, A. et al. A familial childhood-onset relapsing nephrotic syndrome. Kidney Int. 71, 946–951 (2007).
Wasilewska, A. M. et al. Effect of cyclosporin A on proteinuria in the course of glomerulopathy associated with WT1 mutations. Eur. J. Pediatr. 170, 389–391 (2011).
Ransom, R. F., Lam, N. G., Hallett, M. A., Atkinson, S. J. & Smoyer, W. E. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 68, 2473–2483 (2005).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
Coleman, J. E. & Watson, A. R. Hyperlipidaemia, diet and simvastatin therapy in steroid-resistant nephrotic syndrome of childhood. Pediatr. Nephrol. 10, 171–174 (1996).
Sanjad, S. A. et al. Management of hyperlipidemia in children with refractory nephrotic syndrome: the effect of statin therapy. J. Pediatr. 130, 470–474 (1997).
Valdivielso, P. et al. Atorvastatin in dyslipidaemia of the nephrotic syndrome. Nephrology (Carlton) 8, 61–64 (2003).
Ellis, D. et al. Long-term antiproteinuric and renoprotective efficacy and safety of losartan in children with proteinuria. J. Pediatr. 143, 89–97 (2003).
Prescott, W. A. Jr et al. The potential role of HMG-CoA reductase inhibitors in pediatric nephrotic syndrome. Ann. Pharmacother. 38, 2105–2114 (2004).
Conlon, P. J. et al. Spectrum of disease in familial focal and segmental glomerulosclerosis. Kidney Int. 56, 1863–1871 (1999).
Weber, S. et al. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 66, 571–579 (2004).
Jungraithmayr, T. C. et al. Screening for NPHS2 mutations may help predict FSGS recurrence after transplantation. J. Am. Soc. Nephrol. 22, 579–585 (2011).
Kuusniemi, A. M. et al. Plasma exchange and retransplantation in recurrent nephrosis of patients with congenital nephrotic syndrome of the Finnish type (NPHS1). Transplantation 83, 1316–1323 (2007).
Winn, M. P. et al. Focal segmental glomerulosclerosis: a need for caution in live-related renal transplantation. Am. J. Kidney Dis. 33, 970–974 (1999).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
M. P. Winn declares an association with the following company: Athena Diagnostics (consultant). The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Gbadegesin, R., Winn, M. & Smoyer, W. Genetic testing in nephrotic syndrome—challenges and opportunities. Nat Rev Nephrol 9, 179–184 (2013). https://doi.org/10.1038/nrneph.2012.286
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.286
This article is cited by
-
New insights from the genetic work-up in early onset nephrotic syndrome: report from a registry in western India
Pediatric Nephrology (2024)
-
IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome
Pediatric Nephrology (2020)
-
Treatment of nephrotic syndrome: going beyond immunosuppressive therapy
Pediatric Nephrology (2020)
-
Treatment of steroid-resistant nephrotic syndrome in the genomic era
Pediatric Nephrology (2019)
-
An unusual case of nephrotic syndrome in a microcephalic infant: Answers
Pediatric Nephrology (2019)