Abstract
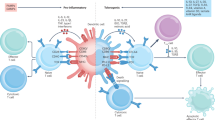
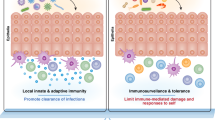
Self tolerance is dependent on mechanisms that operate on T cells and B cells from the earliest stages, that is, from when they first express anti-self-receptors in the primary lymphoid organs of the thymus and bone marrow, all the way through to when they engage with self antigens in the peripheral immune system and within tissues themselves. This continuum of checkpoints and fail-safes ensures that the risk of developing harmful autoimmune diseases remains very small. Certain tissues have a degree of privilege that allows them to mute the immune response against them by mechanisms that are also well represented in cancers. An understanding of the underlying mechanisms of self tolerance is hoped to spawn a new range of therapeutics designed to both reprogram the immune system to avoid long-term intense immunosuppression, and to override the immune system to achieve more effective immunity against cancers and persistent viral infections.
Key Points
-
The immune system is organized so that it is free to react to dangerous microbes while avoiding reactions to self
-
Self tolerance in lymphocytes is organized so that many self-reactive lymphocytes abort soon after they acquire their receptors for antigen
-
Such purging cannot guarantee that self-reactivity will not occur, and other fail-safes operate at numerous checkpoints in the immune response
-
T-regulatory cells and agents that prevent licensing of antigen-presenting dendritic cells are the most amenable to therapeutic exploitation
-
Tissues can adapt to protect themselves against immune attack and can interact with regulatory cells of the innate and adaptive immune systems to create microenvironments that are protected from immune damage
-
Therapeutic exploitation of tolerance mechanisms is constrained by a number of commercial and regulatory issues, so that only part of what we know can ever become drug-worthy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
03 September 2010
In the version of this article initially published online, there was a mistake under the section T-regulatory cells. The errors have been corrected in all electronic versions of the text.
References
Billingham, R. E., Brent, L. & Medawar, P. B. Actively acquired tolerance of foreign cells. Nature 172, 603–606 (1953).
Zinkernagel, R. M. On cross-priming of MHC class I-specific CTL: rule or exception? Eur. J. Immunol. 32, 2385–2392 (2002).
Miller, J. F., Morahan, G. & Allison, J. Immunological tolerance: new approaches using transgenic mice. Immunol. Today 10, 53–57 (1989).
Goodnow, C. C., Crosbie, J., Jorgensen, H., Brink, R. A. & Basten, A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature 342, 385–391 (1989).
Teh, H. S., Kishi, H., Scott, B. & Von Boehmer, H. Deletion of autospecific T cells in T-cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J. Exp. Med. 169, 795–806 (1989).
Schonrich, G. et al. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell 65, 293–304 (1991).
Evans, M. J. Potential for genetic manipulation of mammals. Mol. Biol. Med. 6, 557–565 (1989).
Nelms, K. A. & Goodnow, C. C. Genome-wide ENU mutagenesis to reveal immune regulators. Immunity 15, 409–418 (2001).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat. Genet. 17, 399–403 (1997).
Nagamine, K. et al. Positional cloning of the APECED gene. Nat. Genet. 17, 393–398 (1997).
Klein, L., Klein, T., Ruther, U. & Kyewski, B. CD4 T cell tolerance to human C-reactive protein, an inducible serum protein, is mediated by medullary thymic epithelium. J. Exp. Med. 188, 5–16 (1998).
Bennett, C. L. et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27, 20–21 (2001).
Wildin, R. S. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27, 18–20 (2001).
Kappler, J. W., Roehm, N. & Marrack, P. T cell tolerance by clonal elimination in the thymus. Cell 49, 273–280 (1987).
Dyson, P. J., Knight, A. M., Fairchild, S., Simpson, E. & Tomonari, K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature 349, 531–532 (1991).
Daniels, M. A. et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444, 724–729 (2006).
Anderson, M. S. et al. The cellular mechanism of Aire control of T cell tolerance. Immunity 23, 227–239 (2005).
Nemazee, D. A. & Burki, K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337, 562–566 (1989).
Casellas, R. et al. Contribution of receptor editing to the antibody repertoire. Science 291, 1541–1544 (2001).
Howie, D. et al. MS4A4B is a GITR-associated membrane adapter, expressed by regulatory T cells, which modulates T cell activation. J. Immunol. 183, 4197–4204 (2009).
Miller, J. F. & Mitchell, G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J. Exp. Med. 128, 801–820 (1968).
Claman, H. N. & Chaperon, E. A. Immunologic complementation between thymus and marrow cells—a model for the two-cell theory of immunocompetence. Transplant. Rev. 1, 92–113 (1969).
Metcalf, E. S. & Klinman, N. R. In vitro tolerance induction of neonatal murine B cells. J. Exp. Med. 143, 1327–1340 (1976).
Adams, E., Basten, A. & Goodnow, C. C. Intrinsic B-cell hyporesponsiveness accounts for self-tolerance in lysozyme/anti-lysozyme double-transgenic mice. Proc. Natl Acad. Sci. USA 87, 5687–5691 (1990).
Mitchison, N. A. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular co-operation. Eur. J. Immunol. 1, 18–27 (1971).
Rajewsky, K. The carrier effect and cellular co-operation in the induction of antibodies. Proc. R. Soc. Lond. B Biol. Sci. 176, 385–392 (1971).
Ridge, J. P., Di Rosa, F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478 (1998).
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell co-stimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Xu, J. et al. Mice deficient for the CD40 ligand. Immunity 1, 423–431 (1994).
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Benjamin, R. J. & Waldmann, H. Induction of tolerance by monoclonal antibody therapy. Nature 320, 449–451 (1986).
Gutstein, N. L., Seaman, W. E., Scott, J. H. & Wofsy, D. Induction of immune tolerance by administration of monoclonal antibody to L3T4. J. Immunol. 137, 1127–1132 (1986).
Parker, D. C. et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl Acad. Sci. USA 92, 9560–9564 (1995).
Qin, S. X., Cobbold, S., Benjamin, R. & Waldmann, H. Induction of classical transplantation tolerance in the adult. J. Exp. Med. 169, 779–794 (1989).
Miller, J. F., Morahan, G., Slattery, R. & Allison, J. Transgenic models of T-cell self tolerance and autoimmunity. Immunol. Rev. 118, 21–35 (1990).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Thompson, A. G. & Thomas, R. Induction of immune tolerance by dendritic cells: implications for preventative and therapeutic immunotherapy of autoimmune disease. Immunol. Cell Biol. 80, 509–519 (2002).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Yates, S. F. et al. Induction of regulatory T cells and dominant tolerance by dendritic cells incapable of full activation. J. Immunol. 179, 967–976 (2007).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19, 56–59 (1998).
Mitchell, D. A. et al. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J. Immunol. 168, 2538–2543 (2002).
Goodnow, C. C., Sprent, J., Fazekas de St Groth, B. & Vinuesa, C. G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435, 590–597 (2005).
Walunas, T. L. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413 (1994).
Sanchez-Fueyo, A. et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4, 1093–1101 (2003).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Francisco, L. M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009).
Van den Herik-Oudijk, I. E., Capel, P. J., van der Bruggen, T. & Van de Winkel, J. G. Identification of signaling motifs within human Fc gamma RIIa and Fc gamma RIIb isoforms. Blood 85, 2202–2211 (1995).
Daeron, M. et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity 3, 635–646 (1995).
Crocker, P. R. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 12, 609–615 (2002).
Linsley, P. S. & Nadler, S. G. The clinical utility of inhibiting CD28-mediated co-stimulation. Immunol. Rev. 229, 307–321 (2009).
Kaneko, Y., Nimmerjahn, F. & Ravetch, J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006).
Anthony, R. M., Wermeling, F., Karlsson, M. C. & Ravetch, J. V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl Acad. Sci. USA 105, 19571–19578 (2008).
Duong, B. H. et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J. Exp. Med. 207, 173–187, S171–S174 (2010).
Sakaguchi, S., Takahashi, T. & Nishizuka, Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J. Exp. Med. 156, 1577–1586 (1982).
Kong, Y. M., Giraldo, A. A., Waldmann, H., Cobbold, S. P. & Fuller, B. E. Resistance to experimental autoimmune thyroiditis: L3T4+ cells as mediators of both thyroglobulin-activated and TSH-induced suppression. Clin. Immunol. Immunopathol. 51, 38–54 (1989).
Hall, B. M., Gurley, K. E., Pearce, N. W. & Dorsch, S. E. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. II. Sequential changes in alloreactivity of T cell subsets. Transplantation 47, 1030–1033 (1989).
Qin, S. et al. “Infectious” transplantation tolerance. Science 259, 974–977 (1993).
Powrie, F. & Mason, D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J. Exp. Med. 172, 1701–1708 (1990).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995).
Suri-Payer, E., Amar, A. Z., Thornton, A. M. & Shevach, E. M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160, 1212–1218 (1998).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Chen, W. et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Cobbold, S. P. et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172, 6003–6010 (2004).
Sauer, S. et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl Acad. Sci. USA 105, 7797–7802 (2008).
Haxhinasto, S., Mathis, D. & Benoist, C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008).
Cobbold, S. P. et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl Acad. Sci. USA 106, 12055–12060 (2009).
Waldmann, H., Adams, E. & Cobbold, S. Reprogramming the immune system: co-receptor blockade as a paradigm for harnessing tolerance mechanisms. Immunol. Rev. 223, 361–370 (2008).
Davies, J. D., Leong, L. Y., Mellor, A., Cobbold, S. P. & Waldmann, H. T cell suppression in transplantation tolerance through linked recognition. J. Immunol. 156, 3602–3607 (1996).
Chai, J. G., James, E., Dewchand, H., Simpson, E. & Scott, D. Transplantation tolerance induced by intranasal administration of HY peptides. Blood 103, 3951–3959 (2004).
Graca, L., Cobbold, S. P. & Waldmann, H. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 195, 1641–1646 (2002).
Lin, C. Y., Graca, L., Cobbold, S. P. & Waldmann, H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat. Immunol. 3, 1208–1213 (2002).
Apostolou, I. & von Boehmer, H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 199, 1401–1408 (2004).
Gabrysova, L. & Wraith, D. C. Antigenic strength controls the generation of antigen-specific IL-10-secreting T regulatory cells. Eur. J. Immunol. 40, 1386–1395 (2010).
Streilein, J. W., Masli, S., Takeuchi, M. & Kezuka, T. The eye's view of antigen presentation. Hum. Immunol. 63, 435–443 (2002).
Russell, P. S., Chase, C. M., Colvin, R. B. & Plate, J. M. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J. Exp. Med. 147, 1449–1468 (1978).
Calne, R. Y. et al. Induction of immunological tolerance by porcine liver allografts. Nature 223, 472–476 (1969).
Robertson, N. J. et al. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc. Natl Acad. Sci. USA 104, 20920–20925 (2007).
Driessens, G., Kline, J. & Gajewski, T. F. Co-stimulatory and co-inhibitory receptors in anti-tumor immunity. Immunol. Rev. 229, 126–144 (2009).
Frey, A. B. Myeloid suppressor cells regulate the adaptive immune response to cancer. J. Clin. Invest. 116, 2587–2590 (2006).
Gajewski, T. F. et al. Immune resistance orchestrated by the tumor microenvironment. Immunol. Rev. 213, 131–145 (2006).
Gajewski, T. F. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin. Cancer Res. 13, 5256–5261 (2007).
Rakhmilevich, A. L., North, R. J. & Dye, E. S. Presence of CD4+ T suppressor cells in mice rendered unresponsive to tumor antigens by intravenous injection of irradiated tumor cells. Int. J. Cancer 55, 338–343 (1993).
Curiel, T. J. Regulatory T cells and treatment of cancer. Curr. Opin. Immunol. 20, 241–246 (2008).
Bronte, V. & Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Zea, A. H. et al. Decreased expression of CD3zeta and nuclear transcription factor kappa B in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J. Infect. Dis. 194, 1385–1393 (2006).
Viola, A. & Bronte, V. Metabolic mechanisms of cancer-induced inhibition of immune responses. Semin. Cancer Biol. 17, 309–316 (2007).
Zhang, L., Gajewski, T. F. & Kline, J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 114, 1545–1552 (2009).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009).
Herold, K. C. et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54, 1763–1769 (2005).
Keymeulen, B. et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608 (2005).
Coles, A. J. et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J. Neurol. 253, 98–108 (2006).
Coles, A. J. et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801 (2008).
Kawai, T. et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 358, 353–361 (2008).
Peggs, K. S., Quezada, S. A., Korman, A. J. & Allison, J. P. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr. Opin. Immunol. 18, 206–213 (2006).
Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3, 135–142 (2002).
Tone, M. et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is co-stimulatory for T cells. Proc. Natl Acad. Sci. USA 100, 15059–15064 (2003).
Lu, L. F. et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442, 997–1002 (2006).
Waldmann, H. Immunology: protection and privilege. Nature 442, 987–988 (2006).
de Vries, V. C. et al. Mast cell degranulation breaks peripheral tolerance. Am. J. Transplant. 9, 2270–2280 (2009).
Naesens, M. & Sarwal, M. M. Molecular diagnostics in transplantation. Nat. Rev. Nephrol. doi: 10.1038/nrneph.2010.113.
Hernandez-Fuentes, M. P. & Lechler, R. I. A 'biomarker signature' for tolerance in transplantation. Nat. Rev. Nephrol. doi: 10.1038/nrneph.2010.112.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
H. Waldmann is a co-founder and board member of Tolerx.
Rights and permissions
About this article
Cite this article
Waldmann, H. Tolerance: an overview and perspectives. Nat Rev Nephrol 6, 569–576 (2010). https://doi.org/10.1038/nrneph.2010.108
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.108
This article is cited by
-
Immunomodulatory plasticity of mesenchymal stem cells: a potential key to successful solid organ transplantation
Journal of Translational Medicine (2018)
-
Therapeutic application of T regulatory cells in composite tissue allotransplantation
Journal of Translational Medicine (2017)
-
Unique B7-H1 expression on masticatory mucosae in the oral cavity and trans-coinhibition by B7-H1-expressing keratinocytes regulating CD4+ T cell-mediated mucosal tissue inflammation
Mucosal Immunology (2017)
-
The etiology of glomerulonephritis: roles of infection and autoimmunity
Kidney International (2014)