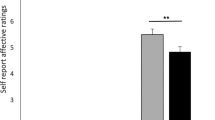
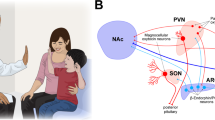
Key Points
-
Placebo effects are effects of the context surrounding medical treatment. They can have meaningfully large impacts on clinical, physiological and brain outcomes.
-
Effects of placebo treatments are consistent across studies from different laboratories. These effects include reduced activity in brain areas associated with pain and negative emotion, and increased activity in the lateral and medial prefrontal cortex, ventral striatum and brainstem.
-
Placebo effects in pain, Parkinson disease, depression and emotion are enabled by engagement of common prefrontal–subcortical motivational systems, but the similarity across domains in the way these systems are engaged has not been directly tested.
-
Meaningfully large placebo effects are likely to require a mixture of both conceptual belief in the placebo and prior experiences of treatment benefit, which engage brain learning processes.
-
In some cases, placebo effects are self-reinforcing, suggesting that they change symptoms in a way that precludes extinction. The mechanisms that drive these effects remain to be uncovered, but doing so could have profound translational implications.
Abstract
Placebo effects are beneficial effects that are attributable to the brain–mind responses to the context in which a treatment is delivered rather than to the specific actions of the drug. They are mediated by diverse processes — including learning, expectations and social cognition — and can influence various clinical and physiological outcomes related to health. Emerging neuroscience evidence implicates multiple brain systems and neurochemical mediators, including opioids and dopamine. We present an empirical review of the brain systems that are involved in placebo effects, focusing on placebo analgesia, and a conceptual framework linking these findings to the mind–brain processes that mediate them. This framework suggests that the neuropsychological processes that mediate placebo effects may be crucial for a wide array of therapeutic approaches, including many drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Committee on Advancing Pain Research, Care, and Education in Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (ed. Institute of Medicine of the National Academies) 1–350 (The National Academies Press, 2011).
Benedetti, F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron 84, 623–637 (2014). This review discusses the pharmacological foundation of many types of placebo effects and addresses the translational and ethical implications of placebo studies.
Walsh, B. T., Seidman, S. N., Sysko, R. & Gould, M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 287, 1840–1847 (2002).
Benedetti, F., Carlino, E. & Pollo, A. How placebos change the patient's brain. Neuropsychopharmacology 36, 339–354 (2011).
Meissner, K. The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Phil. Trans. R. Soc. B 366, 1808–1817 (2011). This review focuses on the evidence that placebos influence autonomic nervous system responses, including effects on gastrointestinal, cardiovascular and pulmonary functions.
Pressman, A., Avins, A. L., Neuhaus, J., Ackerson, L. & Rudd, P. Adherence to placebo and mortality in the Beta Blocker Evaluation of Survival Trial (BEST). Contemp. Clin. Trials 33, 492–498 (2012).
Schenk, L. A., Sprenger, C., Geuter, S. & Buchel, C. Expectation requires treatment to boost pain relief: an fMRI study. Pain 155, 150–157 (2014).
Colloca, L., Lopiano, L., Lanotte, M. & Benedetti, F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol. 3, 679–684 (2004).
Rohsenow, D. J. & Marlatt, G. A. The balanced placebo design: methodological considerations. Addict. Behav. 6, 107–122 (1981).
Kirsch, I. et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 5, e45 (2008).
Flood, A., Lorence, D., Ding, J., McPherson, K. & Black, N. A. The role of expectations in patients' reports of post-operative outcomes and improvement following therapy. Med. Care 31, 1043–1056 (1993).
Goetz, C. G. et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov. Disord. 23, 690–699 (2008).
Wampold, B. E. et al. A meta-analysis of outcome studies comparing bona fide psychotherapies: empiricially,” all must have prizes”. Psychol. Bull. 122, 203–215 (1997).
Kleijnen, J., de Craen, A. J., van Everdingen, J. & Krol, L. Placebo effect in double-blind clinical trials: a review of interactions with medications. Lancet 344, 1347–1349 (1994).
Flaten, M. A., Simonsen, T. & Olsen, H. Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosomat. Med. 61, 250–255 (1999).
Kong, J. et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage 45, 940–949 (2009).
Atlas, L. Y. et al. Dissociable influences of opiates and expectations on pain. J. Neurosci. 32, 8053–8064 (2012). This paper used pharmacological fMRI of remifentanil, an opioid agonist, to examine how placebo effects combine with drug effects during open drug administration and found that placebo analgesia and opioid analgesia have additive, dissociable effects on pain and brain responses.
Atlas, L. Y., Wielgosz, J., Whittington, R. A. & Wager, T. D. Specifying the non-specific factors underlying opioid analgesia: expectancy, attention, and affect. Psychopharmacology 231, 813–823 (2014).
Benedetti, F. et al. The specific effects of prior opioid exposure on placebo analgesia and placebo respiratory depression. Pain 75, 313–319 (1998).
Maren, S., Phan, K. L. & Liberzon, I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428 (2013).
Buchel, C., Geuter, S., Sprenger, C. & Eippert, F. Placebo analgesia: a predictive coding perspective. Neuron 81, 1223–1239 (2014). This review focuses on placebo analgesia from a Bayesian predictive-coding perspective and addresses the relationship between expectations, experience and decision making.
Sterzer, P., Frith, C. & Petrovic, P. Believing is seeing: expectations alter visual awareness. Curr. Biol. 18, R697–R698 (2008).
Summerfield, C. & de Lange, F. P. Expectation in perceptual decision making: neural and computational mechanisms. Nat. Rev. Neurosci. 15, 745–756 (2014).
Summerfield, C. & Egner, T. Expectation (and attention) in visual cognition. Trends Cogn. Sci. 13, 403–409 (2009).
Edelson, M., Sharot, T., Dolan, R. J. & Dudai, Y. Following the crowd: brain substrates of long-term memory conformity. Science 333, 108–111 (2011).
Plassmann, H., O'Doherty, J., Shiv, B. & Rangel, A. Marketing actions can modulate neural representations of experienced pleasantness. Proc. Natl Acad. Sci. USA 105, 1050–1054 (2008).
Hare, T. A., Malmaud, J. & Rangel, A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J. Neurosci. 31, 11077–11087 (2011).
Plassmann, H. & Wager, T. D. in The Interdisciplinary Science of Consumption (eds Kringelbach, M., Knutson, B. & Preston, S.) 219–240 (2014).
Beedie, C. J. & Foad, A. J. The placebo effect in sports performance. Sports Med. 39, 313–329 (2009).
Boot, W. R., Simons, D. J., Stothart, C. & Stutts, C. The pervasive problem with placebos in psychology why active control groups are not sufficient to rule out placebo effects. Persp. Psychol. Sci. 8, 445–454 (2013).
Carlino, E., Frisaldi, E. & Benedetti, F. Pain and the context. Nat. Rev. Rheumatol. 10, 348–355 (2014).
Sherman, R. & Hickner, J. Academic physicians use placebos in clinical practice and believe in the mind–body connection. J. Gen. Intern. Med. 23, 7–10 (2008).
Ochoa, J. L. Chronic pains associated with positive and negative sensory, motor, and vaso-motor manifestations: CPSMV (RSD; CRPS?). Heterogeneous somatic versus psychopathologic origins. Contemp. Neurol. 2, 1–20 (1997).
Barrett, B. et al. Placebo, meaning, and health. Perspect. Biol. Med. 49, 178–198 (2006).
Enck, P., Benedetti, F. & Schedlowski, M. New insights into the placebo and nocebo responses. Neuron 59, 195–206 (2008).
Vase, L., Petersen, G. L., Riley, J. L. & Price, D. D. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain 145, 36–44 (2009).
Vase, L., Riley, J. L. & Price, D. D. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain 99, 443–452 (2002).
Kaptchuk, T. J. et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 336, 999–1003 (2008). This clinical trial of placebo acupuncture found that patients with irritable bowel syndrome improved most when clinicians delivered treatment in a warm, supportive manner, which provides evidence that the patient–care provider relationship can influence treatment outcomes.
Kaptchuk, T. J. et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE 5, e15591 (2010).
Kam-Hansen, S. et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci. Transl Med. 6, 218ra5 (2014).
Rutherford, B. R., Roose, S. P. & Sneed, J. Mind over medicine: the influence of expectations on antidepressant response. J. Am. Psychoanal. Assoc. 57, 456–460 (2009).
Kirsch, I. Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prevent. Treat. 1, Article 2A (1998).
Leuchter, A. F., Hunter, A. M., Tartter, M. & Cook, I. A. Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. Br. J. Psychiatry 205, 443–449 (2014).
Lidstone, S. C. et al. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch. Gen. Psychiatry 67, 857–865 (2010). This study examined placebo effects in patients with PD and found that the strength of expectations for treatment influenced both clinical symptom reduction and striatal dopamine binding.
de la Fuente-Fernandez, R. et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 293, 1164–1166 (2001).
Kemeny, M. E. et al. Placebo response in asthma: a robust and objective phenomenon. J. Allergy Clin. Immunol. 119, 1375–1381 (2007).
Luparello, T., Lyons, H. A., Bleecker, E. R. & McFadden, E. R. Jr. Influences of suggestion on airway reactivity in asthmatic subjects. Psychosomat. Med. 30, 819–825 (1968).
Wechsler, M. E. et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N. Engl. J. Med. 365, 119–126 (2011).
Vase, L., Robinson, M., Verne, G. & Price, D. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain 105, 17–25 (2003).
Avins, A. L. et al. Placebo adherence and its association with morbidity and mortality in the studies of left ventricular dysfunction. J. Gen. Intern. Med. 25, 1275–1281 (2010).
Pollo, A., Vighetti, S., Rainero, I. & Benedetti, F. Placebo analgesia and the heart. Pain 102, 125–133 (2003).
Benedetti, F., Arduino, C. & Amanzio, M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J. Neurosci. 19, 3639–3648 (1999).
Bingel, U. et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl Med. 3, 70ra14 (2011).
Saper, C. B. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469 (2002).
Price, J. Prefrontal cortical networks related to visceral function and mood. Ann. NY Acad. Sci. 877, 383–396 (1999).
Wager, T. D. et al. Brain mediators of cardiovascular responses to social threat, part II: prefrontal–subcortical pathways and relationship with anxiety. Neuroimage 47, 836–851 (2009).
Phelps, E. A. et al. Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 4, 437–441 (2001).
Grings, W. W., Schell, A. M. & Carey, C. A. Verbal control of an autonomic response in a cue reversal situation. J. Exp. Psychol. 99, 215–221 (1973).
Geuter, S. & Büchel, C. Facilitation of pain in the human spinal cord by nocebo treatment. J. Neurosci. 33, 13784–13790 (2013).
Nakamura, Y. et al. Investigating dose-dependent effects of placebo analgesia: a psychophysiological approach. Pain 153, 227–237 (2012).
Eippert, F. et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543 (2009). This study demonstrated, for the first time, that placebo-induced reductions in pain-related fMRI activity are reversible by naloxone.
Benedetti, F., Amanzio, M., Vighetti, S. & Asteggiano, G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J. Neurosci. 26, 12014–12022 (2006).
Johansen, O., Brox, J. & Flaten, M. A. Placebo and nocebo responses, cortisol, and circulating β-endorphin. Psychosomat. Med. 65, 786–790 (2003).
Guo, J. Y. et al. Placebo analgesia affects the behavioral despair tests and hormonal secretions in mice. Psychopharmacology 217, 83–90 (2011).
Benedetti, F. et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 23, 4315–4323 (2003). This multiday study separated placebo effects that depend on conditioning from those that depend on instructions, and found that placebo effects on pain and motor performance in PD reverse immediately with instructions, whereas placebo effects on growth hormone and cortisol mimic pharmacological conditioning.
Crum, A. J., Corbin, W. R., Brownell, K. D. & Salovey, P. Mind over milkshakes: mindsets, not just nutrients, determine ghrelin response. Health Psychol. 30, 424–429 (2011).
Woods, S. C. & Ramsay, D. S. Pavlovian influences over food and drug intake. Behav. Brain Res. 110, 175–182 (2000).
Ader, R. & Cohen, N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science 215, 1534–1536 (1982).
Goebel, M. U. et al. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 16, 1869–1873 (2002).
Schedlowski, M. & Pacheco-Lopez, G. The learned immune response: Pavlov and beyond. Brain Behav. Immun. 24, 176–185 (2010).
Exton, M. S. et al. Behaviorally conditioned immunosuppression in the rat is regulated via noradrenaline and β-adrenoceptors. J. Neuroimmunol. 131, 21–30 (2002).
Pacheco-Lopez, G. et al. Neural substrates for behaviorally conditioned immunosuppression in the rat. J. Neurosci. 25, 2330–2337 (2005).
Ober, K. et al. Plasma noradrenaline and state anxiety levels predict placebo response in learned immunosuppression. Clin. Pharmacol. Ther. 91, 220–226 (2012).
Kamenica, E., Naclerio, R. & Malani, A. Advertisements impact the physiological efficacy of a branded drug. Proc. Natl Acad. Sci. USA 110, 12931–12935 (2013).
Tversky, A. & Kahneman, D. Judgment under uncertainty: heuristics and biases. Science 185, 1124–1131 (1974).
Staudinger, M. R. & Buchel, C. How initial confirmatory experience potentiates the detrimental influence of bad advice. Neuroimage 76, 125–133 (2013).
Wager, T. D., Matre, D. & Casey, K. L. Placebo effects in laser-evoked pain potentials. Brain Behav. Immun. 20, 219–230 (2006).
Martini, M., Lee, M., Valentini, E. & Iannetti, G. Intracortical modulation, and not spinal inhibition, mediates placebo analgesia. Eur. J. Neurosci. 41, 498–504 (2015).
Halperin, E., Russell, A. G., Trzesniewski, K. H., Gross, J. J. & Dweck, C. S. Promoting the Middle East peace process by changing beliefs about group malleability. Science 333, 1767–1769 (2011).
Petrovic, P. et al. Placebo in emotional processing — induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46, 957–969 (2005). This study demonstrated that placebo anxiolytics modulate BOLD responses to emotional images and that these modulations were paralleled by fMRI activation in some of the same brain regions as previously found in placebo analgesia.
Zhang, W., Guo, J., Zhang, J. & Luo, J. Neural mechanism of placebo effects and cognitive reappraisal in emotion regulation. Prog. Neuropsychopharmacol. Biol. Psychiatry 40, 364–373 (2013).
Zhang, W. & Luo, J. The transferable placebo effect from pain to emotion: changes in behavior and EEG activity. Psychophysiology 46, 626–634 (2009). This study, which found that a placebo analgesic also modulated negative affect and EEG responses to unpleasant pictures, represents one of the few studies to formally examine placebo effects across domains.
Schienle, A., Übel, S., Schöngaßner, F., Ille, R. & Scharmüller, W. Disgust regulation via placebo: an fMRI study. Soc. Cogn. Affect. Neurosci. 9, 985–990 (2013).
Schienle, A., Übel, S. & Scharmüller, W. Placebo treatment can alter primary visual cortex activity and connectivity. Neuroscience 263, 125–129 (2014).
Schmidt, L., Braun, E. K., Wager, T. D. & Shohamy, D. Mind matters: placebo enhances reward learning in Parkinson's disease. Nat. Neurosci. 17, 1793–1797 (2014).
Mayberg, H. S. et al. The functional neuroanatomy of the placebo effect. Am. J. Psychiatry 159, 728–737 (2002).
Leuchter, A. F., Cook, I. A., Witte, E. A., Morgan, M. & Abrams, M. Changes in brain function of depressed subjects during treatment with placebo. Am. J. Psychiatry 159, 122–129 (2002).
Apkarian, A. V., Bushnell, M. C., Treede, R. D. & Zubieta, J. K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484 (2005).
Watson, A. et al. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145, 24–30 (2009).
Wager, T. D. et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 303, 1162–1167 (2004). This study used a heat pain model to examine the neural basis of placebo analgesia and was the first fMRI study of placebo analgesia.
Price, D. D., Craggs, J., Verne, G. N., Perlstein, W. M. & Robinson, M. E. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain 127, 63–72 (2007).
Koyama, T., McHaffie, J. G., Laurienti, P. & Coghill, R. C. The subjective experience of pain: where expectations become reality. Proc. Natl Acad. Sci. USA 102, 12950–12955 (2005).
Keltner, J. et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J. Neurosci. 26, 4437–4443 (2006).
Geuter, S., Eippert, F., Attar, C. H. & Büchel, C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage 67, 227–236 (2013).
Wiech, K. et al. Anterior insula integrates information about salience into perceptual decisions about pain. J. Neurosci. 30, 16324–16331 (2010).
Lee, H. F. et al. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain 153, 1301–1310 (2012).
Lu, H.-C. et al. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: a 3T-fMRI study. Pain 148, 75–83 (2009).
Atlas, L. Y., Bolger, N., Lindquist, M. A. & Wager, T. D. Brain mediators of predictive cue effects on perceived pain. J. Neurosci. 30, 12964–12977 (2010).
Kong, J. et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J. Neurosci. 26, 381–388 (2006).
Wager, T. D., Atlas, L. Y., Leotti, L. A. & Rilling, J. K. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J. Neurosci. 31, 439–452 (2011).
Elsenbruch, S. et al. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain 153, 382–390 (2012).
Kong, J. et al. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain 154, 459–467 (2013).
Atlas, L. Y. & Wager, T. D. in Placebo (eds Benedetti, F., Enck, P., Frisaldi, E. & Schedlowski, M.) 37–69 (Springer, 2014).
Koban, L., Ruzic, L. & Wager, T. D. in Placebo and Pain (eds Colloca, L., Flaten, M. A. & Meissner, K.) 89–102 (Academic, 2013).
Amanzio, M., Benedetti, F., Porro, C. A., Palermo, S. & Cauda, F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum. Brain Mapp. 34, 738–752 (2013).
Woo, C.-W., Roy, M., Buhle, J. T. & Wager, T. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol. 13, e1002036 (2015).
Lorenz, J. et al. Cortical correlates of false expectations during pain intensity judgments — a possible manifestation of placebo/nocebo cognitions. Brain Behav. Immun. 19, 283–295 (2005).
Aslaksen, P. M., Bystad, M., Vambheim, S. M. & Flaten, M. A. Gender differences in placebo analgesia: event-related potentials and emotional modulation. Psychosomat. Med. 73, 193–199 (2011).
Colloca, L. et al. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain 139, 306–314 (2009).
Watson, A., El-Deredy, W., Vogt, B. A. & Jones, A. K. Placebo analgesia is not due to compliance or habituation: EEG and behavioural evidence. Neuroreport 18, 771–775 (2007).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Kross, E., Berman, M. G., Mischel, W., Smith, E. E. & Wager, T. D. Social rejection shares somatosensory representations with physical pain. Proc. Natl Acad. Sci. USA 108, 6270–6275 (2011).
Davis, K. D., Taylor, S. J., Crawley, A. P., Wood, M. L. & Mikulis, D. J. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J. Neurophysiol. 77, 3370–3380 (1997).
Lindquist, K. A., Wager, T. D., Kober, H., Bliss-Moreau, E. & Barrett, L. F. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 35, 121–143 (2012).
Woo, C. W. et al. Separate neural representations for physical pain and social rejection. Nat. Commun. 5, 5380 (2014).
Craig, A. D., Chen, K., Bandy, D. & Reiman, E. M. Thermosensory activation of insular cortex. Nat. Neurosci. 3, 184–190 (2000).
Porreca, F., Ossipov, M. H. & Gebhart, G. Chronic pain and medullary descending facilitation. Trends Neurosci. 25, 319–325 (2002).
Gebhart, G. Descending modulation of pain. Neurosci. Biobehav. Rev. 27, 729–737 (2004).
Heinricher, M. & Fields, H. in Wall & Melzack's Textbook of Pain (eds McMahon, S., Koltzenburg, M., Tracey, I. & Turk, D. C.) 129–142 (Elsevier Health Sciences, 2013).
Fields, H. L. State-dependent opioid control of pain. Nat. Rev. Neurosci. 5, 565–575 (2004).
Barbas, H., Saha, S., Rempel-Clower, N. & Ghashghaei, T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 4, 25 (2003).
Keay, K. & Bandler, R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci. Biobehav. Rev. 25, 669–678 (2001).
Wright, J. S. & Panksepp, J. Toward affective circuit-based preclinical models of depression: sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neurosci. Biobehav. Rev. 35, 1902–1915 (2011).
Satpute, A. B. et al. Identification of discrete functional subregions of the human periaqueductal gray. Proc. Natl Acad. Sci. USA 110, 17101–17106 (2013).
Linnman, C., Moulton, E. A., Barmettler, G., Becerra, L. & Borsook, D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 60, 505–522 (2012).
Buhle, J. T. et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc. Cogn. Affect. Neurosci. 8, 609–616 (2013).
Levine, J. D., Gordon, N. C. & Fields, H. L. The mechanism of placebo analgesia. Lancet 2, 654–657 (1978). This study demonstrated that placebo analgesia can be blocked with the opioid antagonist naloxone and was the first to demonstrate a biological mechanism for placebo.
Benedetti, F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 64, 535–543 (1996).
Wager, T. & Scott, D. Placebo effects on human μ-opioid activity during pain. Proc. Natl Acad. Sci. USA 104, 11056–11061 (2007).
Scott, D. J. et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 65, 220–231 (2008).
Zubieta, J. et al. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J. Neurosci. 25, 7754–7762 (2005).
Peciña, M. et al. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology 38, 639–646 (2013).
Eippert, F., Finsterbusch, J., Bingel, U. & Büchel, C. Direct evidence for spinal cord involvement in placebo analgesia. Science 326, 404 (2009). This study used fMRI to image the spinal cord and found that spinal responses to noxious stimuli are modulated with placebo, which implicates descending modulation.
Matre, D., Casey, K. L. & Knardahl, S. Placebo-induced changes in spinal cord pain processing. J. Neurosci. 26, 559–563 (2006).
Goffaux, P., de Souza, J., Potvin, S. & Marchand, S. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients. Pain 145, 18–23 (2009).
Petrovic, P., Kalso, E., Petersson, K. M. & Ingvar, M. Placebo and opioid analgesia — imaging a shared neuronal network. Science 295, 1737–1740 (2002). This PET study was the first to use neuroimaging to investigate mechanisms of the placebo response and found that both placebo analgesia and opioid analgesia induce changes in blood flow in the rostral ACC.
Craggs, J. G., Price, D. D., Perlstein, W. M., Verne, G. N. & Robinson, M. E. The dynamic mechanisms of placebo induced analgesia: evidence of sustained and transient regional involvement. Pain 139, 660–669 (2008).
Lui, F. et al. Neural bases of conditioned placebo analgesia. Pain 151, 816–824 (2010).
Bingel, U., Lorenz, J., Schoell, E., Weiller, C. & Buchel, C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120, 8–15 (2006).
Borckardt, J. J. et al. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology 105, 557–562 (2006).
Krummenacher, P., Candia, V., Folkers, G., Schedlowski, M. & Schonbachler, G. Prefrontal cortex modulates placebo analgesia. Pain 148, 368–374 (2010).
Stein, N., Sprenger, C., Scholz, J., Wiech, K. & Bingel, U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain 153, 2210–2217 (2012).
Zhang, Y. Q., Tang, J. S., Yuan, B. & Jia, H. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain 72, 127–135 (1997).
Zhang, S., Tang, J. S., Yuan, B. & Jia, H. Electrically-evoked inhibitory effects of the nucleus submedius on the jaw-opening reflex are mediated by ventrolateral orbital cortex and periaqueductal gray matter in the rat. Neuroscience 92, 867–875 (1999).
Johansen, J. P. et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl Acad. Sci. USA 107, 12692–12697 (2010).
Helmstetter, F. J., Tershner, S. A., Poore, L. H. & Bellgowan, P. S. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Res. 779, 104–118 (1998).
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Kober, H. et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc. Natl Acad. Sci. USA 107, 14811–14816 (2010).
Zaki, J., Schirmer, J. & Mitchell, J. P. Social influence modulates the neural computation of value. Psychol. Sci. 22, 894–900 (2011).
Demos, K. E., Heatherton, T. F. & Kelley, W. M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 32, 5549–5552 (2012).
Berridge, K. C. & Robinson, T. E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 28, 309–369 (1998).
Wager, T. D., Hughes, B., Davidson, M., Lindquist, M. L. & Ochsner, K. N. Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050 (2008).
Navratilova, E. & Porreca, F. Reward and motivation in pain and pain relief. Nat. Neurosci. 17, 1304–1312 (2014).
Schwartz, N. et al. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345, 535–542 (2014).
Metz, A. E., Yau, H.-J., Centeno, M. V., Apkarian, A. V. & Martina, M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl Acad. Sci. USA 106, 2423–2428 (2009).
Baliki, M. N. et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 15, 1117–1119 (2012).
Schweinhardt, P., Seminowicz, D. A., Jaeger, E., Duncan, G. H. & Bushnell, M. C. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J. Neurosci. 29, 4882–4887 (2009).
Scott, D. J. et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55, 325–336 (2007).
Wanigasekera, V. et al. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc. Natl Acad. Sci. USA 109, 17705–17710 (2012).
Zhang, W., Qin, S., Guo, J. & Luo, J. A follow-up fMRI study of a transferable placebo anxiolytic effect. Psychophysiology 48, 1119–1128 (2011).
Benedetti, F. et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat. Neurosci. 7, 587–588 (2004).
Drevets, W. C. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827 (1997).
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005).
Petrovic, P. et al. A prefrontal non-opioid mechanism in placebo analgesia. Pain 150, 59–65 (2010).
Ellingsen, D.-M. et al. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc. Natl Acad. Sci. USA 110, 17993–17998 (2013).
Kessner, S., Sprenger, C., Wrobel, N., Wiech, K. & Bingel, U. Effect of oxytocin on placebo analgesia: a randomized study. JAMA 310, 1733–1735 (2013).
Rahnev, D., Lau, H. & de Lange, F. P. Prior expectation modulates the interaction between sensory and prefrontal regions in the human brain. J. Neurosci. 31, 10741–10748 (2011).
Kok, P., Brouwer, G. J., van Gerven, M. A. & de Lange, F. P. Prior expectations bias sensory representations in visual cortex. J. Neurosci. 33, 16275–16284 (2013).
Flaten, M. A., Aslaksen, P. M., Lyby, P. S. & Bjorkedal, E. The relation of emotions to placebo responses. Phil. Trans. R. Soc. B 366, 1818–1827 (2011).
Roy, M., Shohamy, D. & Wager, T. D. Ventromedial prefrontal–subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 16, 147–156 (2012).
Chib, V. S., Rangel, A., Shimojo, S. & O'Doherty, J. P. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J. Neurosci. 29, 12315–12320 (2009).
Hare, T. A., Camerer, C. F. & Rangel, A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324, 646–648 (2009).
Falk, E. B., Berkman, E. T., Whalen, D. & Lieberman, M. D. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychol. 30, 177–185 (2011).
Wager, T. et al. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397 (2013).
Rescorla, R. A. Pavlovian conditioning. It's not what you think it is. Am. Psychol. 43, 151–160 (1988).
Gallistel, C. R. & Matzel, L. D. The neuroscience of learning: beyond the Hebbian synapse. Annu. Rev. Psychol. 64, 169–200 (2013).
Schoenbaum, G., Roesch, M. R., Stalnaker, T. A. & Takahashi, Y. K. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat. Rev. Neurosci. 10, 885–892 (2009).
Kirsch, I., Lynn, S. J., Vigorito, M. & Miller, R. R. The role of cognition in classical and operant conditioning. J. Clin. Psychol. 60, 369–392 (2004).
Stewart-Williams, S. & Podd, J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol. Bull. 130, 324–340 (2004).
Kirsch, I. Response expectancy as a determinant of experience and behavior. Am. Psychol. 40, 1189–1202 (1985).
Voudouris, N. J., Peck, C. L. & Coleman, G. Conditioned placebo responses. J. Pers. Soc. Psychol. 48, 47–53 (1985).
Wickramasekera, I. A conditioned response model of the placebo effect; predictions from the model. Biofeedback Self Regul. 5, 5–18 (1980).
Carlino, E. et al. Role of explicit verbal information in conditioned analgesia. Eur. J. Pain 19, 546–553 (2015).
Montgomery, G. H. & Kirsch, I. Classical conditioning and the placebo effect. Pain 72, 107–113 (1997).
Wendt, L. et al. Placebo-induced immunosuppression in humans: role of learning and expectation. Brain Behav. Immun. 29, S17 (2013).
Benedetti, F., Amanzio, M., Baldi, S., Casadio, C. & Maggi, G. Inducing placebo respiratory depressant responses in humans via opioid receptors. Eur. J. Neurosci. 11, 625–631 (1999).
Morton, D. L., Watson, A., El-Deredy, W. & Jones, A. K. Reproducibility of placebo analgesia: effect of dispositional optimism. Pain 146, 194–198 (2009).
Morton, D. L., Brown, C. A., Watson, A., El-Deredy, W. & Jones, A. K. Cognitive changes as a result of a single exposure to placebo. Neuropsychologia 48, 1958–1964 (2010).
Doll, B. B., Jacobs, W. J., Sanfey, A. G. & Frank, M. J. Instructional control of reinforcement learning: a behavioral and neurocomputational investigation. Brain Res. 1299, 74–94 (2009).
Biele, G., Rieskamp, J., Krugel, L. K. & Heekeren, H. R. The neural basis of following advice. PLoS Biol. 9, e1001089 (2011).
Li, J., Delgado, M. R. & Phelps, E. A. How instructed knowledge modulates the neural systems of reward learning. Proc. Natl Acad. Sci. USA 108, 55–60 (2011).
Vase, L., Robinson, M., Verne, G. & Price, D. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain 115, 338–347 (2005).
Kirsch, I. & Henry, D. Extinction versus credibility in the desensitization of speech anxiety. J. Consult. Clin. Psychol. 45, 1052–1059 (1977).
Cliffer, K. D., Burstein, R. & Giesler, G. J. Jr. Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J. Neurosci. 11, 852–868 (1991).
Willis, W. D. & Westlund, K. N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 14, 2–31 (1997).
Bandler, R., Keay, K. A., Floyd, N. & Price, J. Central circuits mediating patterned autonomic activity during active versus passive emotional coping. Brain Res. Bull. 53, 95–104 (2000).
Watkins, L. R. & Mayer, D. J. Organization of endogenous opiate and nonopiate pain control systems. Science 216, 1185–1192 (1982).
Altier, N. & Stewart, J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 65, 2269–2287 (1999).
Gear, R. W., Aley, K. O. & Levine, J. D. Pain-induced analgesia mediated by mesolimbic reward circuits. J. Neurosci. 19, 7175–7181 (1999).
Helmstetter, F. J. Stress-induced hypoalgesia and defensive freezing are attenuated by application of diazepam to the amygdala. Pharmacol. Biochem. Behav. 44, 433–438 (1993).
Butler, R. K. & Finn, D. P. Stress-induced analgesia. Prog. Neurobiol. 88, 184–202 (2009).
Yang, J. et al. Central oxytocin enhances antinociception in the rat. Peptides 28, 1113–1119 (2007).
Lund, I. et al. Repeated massage-like stimulation induces long-term effects on nociception: contribution of oxytocinergic mechanisms. Eur. J. Neurosci. 16, 330–338 (2002).
Yang, J. et al. Oxytocin in the periaqueductal gray participates in pain modulation in the rat by influencing endogenous opiate peptides. Peptides 32, 1255–1261 (2011).
Watkins, L. R., Kinscheck, I. B. & Mayer, D. J. Potentiation of opiate analgesia and apparent reversal of morphine tolerance by proglumide. Science 224, 395–396 (1984).
Wiertelak, E. P., Maier, S. F. & Watkins, L. R. Cholecystokinin antianalgesia: safety cues abolish morphine analgesia. Science 256, 830–833 (1992).
Dum, J. & Herz, A. Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacol. Biochem. Behav. 21, 259–266 (1984).
Rodgers, R. J. & Hendrie, C. A. Social conflict activates status-dependent endogenous analgesic or hyperalgesic mechanisms in male mice: effects of naloxone on nociception and behaviour. Physiol. Behav. 30, 775–780 (1983).
Langford, D. J. et al. Varying perceived social threat modulates pain behavior in male mice. J. Pain 12, 125–132 (2011).
Shyu, B. C., Sikes, R. W., Vogt, L. J. & Vogt, B. A. Nociceptive processing by anterior cingulate pyramidal neurons. J. Neurophysiol. 103, 3287–3301 (2010).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Moerman, D. E. Meaning, Medicine and the 'Placebo Effect' (Cambridge Univ. Press, 2002).
Benedetti, F., Durando, J. & Vighetti, S. Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. Pain 155, 921–928 (2014).
Orne, T. M. On the social psychology of the psychological experiment: with particular reference to demand characteristics and their implications. Am. Psychol. 17, 776–783 (1962).
Xie, J. Y. et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J. Neurosci. 25, 409–416 (2005).
Acknowledgements
The authors thank J. Sills and E. Hitchcock for research support, the members of the Cognitive and Affective Neuroscience Lab, S. Maier and L. Watkins for helpful discussions, and L. Ruzic for help with the summary in Figure 3. This work was funded by grants NIMH 2R01MH076136 and R01DA027794 (to T.D.W.). This work was also supported in part by the Intramural Research Program of the US National Institutes of Health's National Center for Complementary and Integrative Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1
Supplementary information S1 (table) (PDF 214 kb)
Supplementary information S2 (box)
Neuroimaging studies included in Figure 3 (PDF 170 kb)
Glossary
- Context
-
The combination of all of the elements surrounding a given event that can be psychologically meaningful, including interpersonal dynamics, situational features owing to a place or location, memories, goals for the future and internal body or brain states.
- Cues
-
Stimuli that signify the occurrence, or evoke a representation, of another stimulus or internal experience.
- Emotions
-
Coordinated responses to biologically relevant events (such as threats and opportunities) that involve changes in multiple systems, including peripheral physiology.
- Nocebo effects
-
Deleterious outcomes (for example, an increase in pain or an increase in negative side effects) owing to beliefs about the treatment context.
- Placebo responders
-
Individuals who show an improvement in symptoms after receiving inert treatments (that is, placebos).
- Placebo analgesia
-
A reduction in pain that can be attributed to the treatment context.
- Response conditioning
-
The process of associating neutral stimuli with biologically meaningful outcomes, through which neutral stimuli may begin to induce anticipatory responses that are associated with the outcomes themselves.
- Expectancy
-
A conscious, conceptual belief about the future occurrence of an event. It is a subclass of predictive processes, which may be conscious or unconscious.
- Analgesia
-
Pain relief, which can be caused by many factors, including medical treatments (for example, opioid analgesia), features of the treatment context (placebo analgesia) and affective states (for example, stress-induced analgesia).
- Nociceptive
-
Receiving input from stimuli that can cause damage to tissues.
- Descending pain modulation systems
-
Endogenous, biological mechanisms for suppressing ascending nociceptive information at the level of the spinal cord.
- Pre-cognitive associations
-
Links between events and/or objects that exist outside conscious awareness. These links are generally created through conditioning procedures or innate (evolutionarily afforded) associations.
- Conceptual processes
-
Processes that depend on an interpretation of the situational context and its relationship to prior information (for example, memories and rules), including interoceptive cues from the body, and which can be updated in response to verbally presented or symbolic information.
- Schema
-
A conceptual, 'situational' pattern — inferred from a combination of sensory cues, internal motivation, interoceptive information and thoughts — that can activate scripts that guide behaviour based on the nature of the situation rather than any single cue.
- Attributions
-
Inferred causality; the process of assigning an observed effect (for example, a symptom) to an underlying cause or mechanism.
Rights and permissions
About this article
Cite this article
Wager, T., Atlas, L. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 16, 403–418 (2015). https://doi.org/10.1038/nrn3976
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3976