Abstract
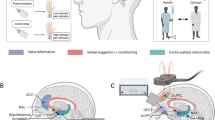
Significant clinical improvement is often observed in patients who receive placebo treatment in randomized double-blind placebo-controlled trials. While a proportion of this “improvement” reflects experimental design limitations (e.g., reliance on subjective outcomes, unbalanced groups, reporting biases), some of it reflects genuine improvement corroborated by physiological change. Converging evidence across diverse medical conditions suggests that clinically-relevant benefits from placebo treatment are associated with the activation of brain reward circuits. In parallel, evidence has accumulated showing that such benefits are facilitated by clinicians that demonstrate warmth and proficiency during interactions with patients. Here, we integrate research on these neural and social aspects of placebo effects with evidence linking oxytocin and social reward to advance a neurobiological account for the social facilitation of placebo effects. This account frames oxytocin as a key mediator of treatment success across a wide-spectrum of interventions that increase social connectedness, thereby providing a biological basis for assessing this fundamental non-specific element of medical care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Notes
More broadly, an individual’s overall level of “social connectedness” is an important predictor of well-being, morbidity, and mortality. Social connectedness can be measured by assessing structural, functional, and/or qualitative aspects of an individual’s relationships (e.g., the size of their social network, their access to social support, and affective valences), and is increased by forging new social connections of the kind described here [46, 47].
References
Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14.
De la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–6.
Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci. 2005;25:7754–62.
Wager TD, Scott DJ, Zubieta JK. Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–61.
Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 2007;55:325–36.
Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–31.
Peciña M, Zubieta JK. Molecular mechanisms of placebo responses in humans. Mol Psychiatry. 2015;20:416–23.
Peciña M, Sikora M, Avery ET, Heffernan J, Peciña S, Mickey BJ, et al. Striatal dopamine D2/3 receptor-mediated neurotransmission in major depression: Implications for anhedonia, anxiety and treatment response. Eur Neuropsychopharmacol. 2017;27:977–86.
Howe LC, Goyer JP, Crum AJ. Harnessing the placebo effect: exploring the influence of physician characteristics on placebo response. Heal Psychol. 2017;36:1074–82.
Pollo A, Torre E, Lopiano L, Rizzone M, Lanotte M, Cavanna A, et al. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. Neuroreport 2002;13:1383–6.
Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN. Placebo response in asthma: A robust and objective phenomenon. J Allergy Clin Immunol. 2007;119:1375–81.
Kamenica E, Naclerio R, Malani A. Advertisements impact the physiological efficacy of a branded drug. Proc Natl Acad Sci USA. 2013;110:12931–5.
Zunhammer M, Spisák T, Wager TD, Bingel U, Atlas L, Benedetti F, et al. Meta-analysis of neural systems underlying placebo analgesia from individual participant fMRI data. Nat Commun. 2021;12:1–11.
Benedetti F Placebo effects: understanding the other side of medical care. third. Oxford: Oxford University Press; 2021.
Rief W, Shedden-Mora MC, Laferton JAC, Auer C, Petrie KJ, Salzmann S, et al. Preoperative optimization of patient expectations improves long-term outcome in heart surgery patients: Results of the randomized controlled PSY-HEART trial. BMC Med. 2017;15:1–13.
Evers AWM, Colloca L, Blease C, Gaab J, Jensen KB, Atlas LY, et al. What should clinicians tell patients about Placebo and Nocebo effects? practical considerations based on expert consensus. Psychother Psychosom. 2020;90:49–56.
Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosom Med. 2011;73:598–603.
Corsi N, Colloca L. Placebo and nocebo effects: the advantage of measuring expectations and psychological factors. Front Psychol. 2017;8:308.
Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci. 2017;114:8119–24.
Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. fourth. New York: Springer; 2010.
Kaptchuk TJ. Powerful placebo: The dark side of the randomised controlled trial. Lancet 1998;351:1722–5.
Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–90.
Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–23.
Kirsch I. Response expectancy as a determinant of experience and behavior. Am Psychol. 1985;40:1189–202.
Lyerly SB, Ross S, Krugman AD, Clyde DJ. Drugs and placebos: the effects of instructions upon performance and mood under amphetamine sulfate and chloral hydrate. J Abnorm Soc Psychol. 1964;68:321–7.
Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation- activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–94.
Koban L, Kross E, Woo CW, Ruzic L, Wager TD. Frontal-brainstem pathways mediating placebo effects on social rejection. J Neurosci. 2017;37:3621–31.
Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–7.
Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;312:654–7.
Goetz CG, Wuu J, McDermott MP, Adler CH, Fahn S, Freed CR, et al. Placebo response in Parkinson’s disease: Comparisons among 11 trials covering medical and surgical interventions. Mov Disord. 2008;23:690–9.
Masi A, Lampit A, Glozier N, Hickie IB, Guastella AJ. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectru disorder: a meta-analysis. Transl Psychiatry. 2015;5:e640.
Kirsch I. Placebo effect in the treatment of depression and anxiety. Front Psychiatry. 2019;10:1–9.
Schafer SM, Colloca L, Wager TD. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. J Pain. 2015;16:412–20.
Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain 2009;144:28–34.
De la Fuente-Fernández R. The placebo-reward hypothesis: dopamine and the placebo effect. Park Relat Disord. 2009;15:72–74.
De la Fuente-Fernández R, Phillips AG, Zamburlini M, Sossi V, Calne DB, Ruth TJ, et al. Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res. 2002;136:359–63.
Strafella AP, Ko JJ, Monchi O. Therapeutic application of TMS in PD: the contribution of expectation. Neuroimage 2006;31:1666–72.
Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:1–5.
Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18:165–71.
Crum AJ, Leibowitz KA, Verghese A. Making mindset matter. BMJ 2017;356:6674.
Espay AJ, Norris MM, Eliassen JC, Smith MS, Banks C, Allendorfer JB, et al. Placebo effect of medication cost in Parkinson disease. Neurology 2015;84:794–802.
Czerniak E, Biegon A, Ziv A, Karnieli-Miller O, Weiser M, Alon U, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. 2016;7:1–10.
Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. Bmj 2008;336:999–1003.
Howe LC, Leibowitz KA, Crum AJ. When your doctor “Gets it” and “Gets you”: the critical role of competence and warmth in the patient-provider interaction. Front Psychiatry. 2019;10:1–22.
Rakel D, Barrett B, Zhang Z, Hoeft T, Chewning B, Marchand L, et al. Perception of empthay in the therapeutic encounter: effects on the common cold. Patient Educ Counc. 2011;85:390–7.
Seppala E, Rossomando T, Doty JR. Social connection and compassion: important predictors of health and well-being. Soc Res. 2013;80:411–30.
Holt-Lunstad J. The major health implications of social connection. Curr Dir Psychol Sci. 2021;30:251–9.
Enck P, Klosterhalfen S. The story of O–Is oxytocin the mediator of the placebo response? Neurogastroenterol Motil. 2009;21:347–50.
Benedetti F The patient’s brain: the neuroscience behind the doctor-patient relationship. Oxford University Press; 2010.
Jurek B, Neumann ID. The oxytocin receptor: From intracellular signaling to behavior. Physiol Rev. 2018;98:1805–908.
Carmichael MS, Warburton VL, Dixen J, Davidson JM. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav. 1994;23:59–79.
Fuchs AR, Fuchs F. Endocrinology of human parturition: a review. Br J Obstet Gynaecol. 1984;91:948–67.
Uvnäs-Moberg K, Prime DK. Oxytocin effects in mothers and infants during breatfeeding. Infant. 2013;9:201–6.
Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc R Soc B. 2010;277:2661–6.
Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, et al. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science. 2015;348:333–6.
Odendaal JSJ, Meintjes RA. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet J. 2003;165:296–301.
Spengler FB, Scheele D, Marsh N, Kofferath C, Flach A, Schwarz S, et al. Oxytocin facilitates reciprocity in social communication. Soc Cogn Affect Neurosci. 2017;12:1325–33.
Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, et al. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci. 2014;111:12258–63.
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 2005;435:673–6.
Declerck CH, Boone C, Pauwels L, Vogt B, Fehr E. A registered replication study on oxytocin and trust. Nat Hum Behav. 2020;4:646–55.
Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:1–5.
Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm Behav. 2010;57:368–74.
De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science (80-). 2010;328:1408–11.
Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves ‘mind-reading’ in humans. Biol Psychiatry. 2007;61:731–3.
Shahrestani S, Kemp AH, Guastella AJ. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology 2013;38:1929–36.
Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5.
Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychol Med. 2013;43:1747–53.
Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11.
Dölen G, Malenka RC. The emerging role of nucleus accumbens oxytocin in social cognition. Biol Psychiatry. 2014;76:354–5.
Young KA, Liu Y, Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol Part C. 2008;148:401–10.
Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressing receptor research in rodents and primates, translational implications. J Neuroendocrinol. 2016;28 https://doi.org/10.1111/jne.12382.
Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–8.
Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47.
Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013;501:179–84.
Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O, et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA. 2013;110:20308–13.
Striepens N, Matusch A, Kendrick KM, Mihov Y, Elmenhorst D, Becker B, et al. Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology 2014;39:74–87.
Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, et al. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74:172–9.
Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–7.
Cho MM, Devries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci. 1999;113:1071–9.
Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–90.
Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci. 2000;114:173–83.
Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 2003;121:537–44.
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell 2014;157:1535–51.
Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406–11.
Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE. Activation of oxytocin receptors, but not arginine-vasopressin (AVP) receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 2016;74:164–72.
Le Merrer J, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–412.
Nelson EE, Panksepp J. Brain substrates of infant–mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–52.
Trezza V, Damsteegt R, Marijke Achterberg EJ, Vanderschuren LJMJ. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 2011;31:6362–70.
Douglas AJ, Neumann I, Meeren HKM, Leng G, Johnstone LE, Munro G, et al. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J Neurosci. 1995;15:5049–57.
Clarke G, Wood P, Merrick L, Lincoln DW. Opiate inhibition of peptide release from the neurohumoral terminals of hypothalamic neurones. Nature 1979;282:746–8.
Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned μ, δ and κ receptors and their endogenous ligands: Evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98.
You ZD, Li JH, Song CY, Wang CH, Lu CL. Chronic morphine treatment inhibits oxytocin synthesis in rats. Neuroreport 2000;11:3113–6.
Dal Monte O, Piva M, Anderson KM, Tringides M, Holmes AJ, Chang SWC. Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. Proc Natl Acad Sci USA. 2017;114:5247–52.
Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the μ-opioid receptor gene. Science. 2004;304:1983–6.
Cinque C, Pondiki S, Oddi D, Di Certo MG, Marinelli S, Troisi A, et al. Modeling socially anhedonic syndromes: Genetic and pharmacological manipulation of opioid neurotransmission in mice. Transl Psychiatry. 2012;2:1–7.
Wöhr M, Moles A, Schwarting RKW, D’Amato FR. Lack of social exploratory activation in male μ-opioid receptor KO mice in response to playback of female ultrasonic vocalizations. Soc Neurosci. 2011;6:76–87.
Gigliucci V, Leonzino M, Busnelli M, Luchetti A, Palladino VS, D’Amato FR, et al. Region specific up-regulation of oxytocin receptors in the opioid Oprm1 -/- mouse model of autism. Front Pediatr. 2014;2:1–12.
Meguro Y, Miyano K, Hirayama S, Yoshida Y, Ishibashi N, Ogino T, et al. Neuropeptide oxytocin enhances μ opioid receptor signaling as a positive allosteric modulator. J Pharm Sci. 2018;137:67–75.
Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci USA. 1990;87:230–4.
Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113.
MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res. 2004;1018:78–85.
Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, et al. Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. Elife 2018;7:1–22.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–38.
Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015;86:646–64.
Herbert J. Peptides in the limbic system: neurochemical codes for co-ordinated adaptive responses to behavioural and physiological demand. Prog Neurobiol. 1993;41:723–91.
Csiffáry A, Ruttner Z, Tóth Z, Palkovits M. Oxytocin nerve fibers innervate Beta-endorphin neurons in the arcuate nucleus of the rat hypothalamus. Neuroendocrinology 1992;56:429–35.
Pandit R, Omrani A, Luijendijk MCM, De Vrind VAJ, Van Rozen AJ, Ophuis RJAO, et al. Melanocortin 3 receptor signaling in midbrain dopamine neurons increases the motivation for food reward. Neuropsychopharmacology 2016;41:2241–51.
Beaulieu J, Champagne D, Drolet G. Enkephalin innervation of the paraventricular nucleus of the hypothalamus: distribution of fibers and origins of input. J Chem Neuroanat. 1996;10:79–92.
Douglas AJ, Bicknell RJ, Leng G, Russell JA, Meddle SL. β-Endorphin cells in the arcuate nucleus: projections to the supraoptic nucleus and changes in expression during pregnancy and parturition. J Neuroendocrinol. 2002;14:768–77.
Flückiger C, Re ACDel, Wampold BE, Symonds D, Horvath AO. How central is the alliance in psychotherapy? A multilevel longitudinal meta-analysis. J Couns Psychol. 2012;59:10–17.
Smith ML, Glass GV. Meta-analysis of psychotherapy outcome studies. Am Psychol. 1977;32:752–60.
Strupp HH, Hadley SW. Specific vs nonspecific factors in psychotherapy: a controlled study of outcome. Arch Gen Psychiatry. 1979;36:1125–36.
Ackerman SJ, Hilsenroth MJ. A review of therapist characteristics and techniques positively impacting the therapeutic alliance. Clin Psychol Rev. 2003;23:1–33.
Ardito RB, Rabellino D. Therapeutic alliance and outcome of psychotherapy: historical excursus, measurements, and prospects for research. Front Psychol. 2011;2:1–11.
Horvath AO, Luborsky L. The role of the therapeutic alliance in psychotherapy. J Consult Clin Psychol. 1993;61:561–73.
Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder. Jama 2010;303:1929.
Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2007. 2007. https://doi.org/10.1002/14651858.CD003388.pub3.
Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of CBT: a review of meta-analyses. Cogn Ther Res. 2012;36:427–40.
López-lópez JA, Davies SR, Caldwell DM, Churchill R, Peters TJ, Tallon D, et al. The process and delivery of CBT for depression in adults: a systematic review and network meta-analysis. Psychol Med. 2019;49:1937–47.
Kirsch I. Changing expectations: a key to effective psychotherapy. Pacific Grove, CA: Brooks/Cole.; 1990.
King BH, Dukes K, Donnelly CL, Sikich L, McCracken JT, Scahill L, et al. Baseline factors predicting placebo response to treatment in children and adolescents with autism spectrum disorders: A multisite randomized clinical trial. JAMA Pediatr. 2013;167:1045–52.
Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord. 2003;33:427–33.
Cochran DM, Fallon D, Hill M, Frazier JA. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry. 2013;21:219–47.
Herpertz SC, Bertsch K. A new perspective on the pathophysiology of borderline personality disorder: a model of the role of oxytocin. Am J Psychiatry. 2015;172:840–51.
Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–98.
Karelina K, Devries AC. Modeling social influences on human health. Psychosom Med. 2011;73:67–74.
Knox SS, Uvnäs-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology 1998;23:877–90.
Peciña M, Bohnert ASB, Sikora M, Avery ET, Langenecker SA, Mickey BJ, et al. Association between placebo-activated neural systems and antidepressant responses neurochemistry of placebo effects in major depression. JAMA Psychiatry. 2015;72:1087–94.
Kessner S, Sprenger C, Wrobel N, Wiech K, Bingel U. Effect of oxytocin on placebo analgesia: a randomized study. JAMA - J Am Med Assoc. 2013;310:1733–5.
Zhao W, Becker B, Yao S, Ma X, Kou J, Kendrick KM. Oxytocin enhancement of the placebo effect may be a novel therapy for working memory impairments. Psychother Psychosom. 2019;88:125–6.
Skvortsova A, Veldhuijzen DS, van Middendorp H, Colloca L, Evers AWM. Effects of oxytocin on placebo and nocebo effects in a pain conditioning paradigm: a randomized controlled trial. J Pain. 2020;21:430–9.
Liu C, Huang Y, Chen L, Yu R. Lack of evidence for the effect of oxytocin on placebo analgesia and nocebo hyperalgesia. Psychother Psychosom. 2020;89:185–7.
Colloca L, Pine DS, Ernst M, Miller FG, Grillon C. Vasopressin boosts placebo analgesic effects in women: a randomized trial. Biol Psychiatry. 2016;79:794–802.
Skvortsova A, Veldhuijzen DS, Van Middendorp H, Van Den Bergh O, Evers AWM. Enhancing placebo effects in somatic symptoms through oxytocin. Psychosom Med. 2018;80:353–60.
Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4.
Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010;65:768–79.
Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci USA. 2006;103:7889–94.
Rilling JK, Li T, Chen X, Gautam P, Haroon E, Thompson RR. Arginine vasopressin effects on subjective Judgments and neural responses to same and other-sex Faces in men and women. Front Endocrinol. 2017;8:200.
Chen X, Hackett PD, DeMarco AC, Feng C, Stair S, Haroon E, et al. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav. 2016;10:581–93.
Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, et al. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 2015;9:754–64.
Enck P, Klosterhalfen S. Does sex/gender play a role in placebo and nocebo effects? Conflicting evidence from clinical trials and experimental studies. Front Neurosci. 2019;13:160.
Vambheim SM, Flaten MA. A systematic review of sex differences in the placebo and the nocebo effect. J Pain Res. 2017;10:1831–9.
Becker B, Zhao W, Kendrick KM. Reply to the letter to the editor: ‘lack of evidence for the effect of oxytocin on placebo analgesia and nocebo hyperalgesia’. Psychother Psychosom. 2020;89:188.
Kaptchuk TJ. Open-label placebo reflections on a research agenda. Perspect Biol Med. 2018;61:311–34.
Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–9.
Froemke RC, Young, LJ. Oxytocin, Neural Plasticity, and Social Behavior. Annu Rev Neurosci. 2021;44:359–81.
Luo R, Xu L, Zhao W, Ma X, Xu X, Kou J, et al. Oxytocin facilitation of acceptance of social advice is dependent upon the perceived trustworthiness of individual advisors. Psychoneuroendocrinology 2017;83:1–8.
Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012;73:553–66.
Lewis EM, Stein-O’Brien GL, Patino AV, Nardou R, Grossman CD, Brown M, et al. Parallel social information processing circuits are differentially impacted in autism. Neuron 2020;108:659–675.e6.
Acknowledgements
We are grateful to Olena Zyga for her input on the clinical implications of social connectedness on an early draft of this manuscript. This work was supported by National Institute of Mental Health Grant K01MH122730 (DLB), a seed grant from the Wu Tsai Neuroscience Institute at Stanford University (DLB & KPJ), a Stanford School of Medicine Dean’s fellowship (EI), and the Stanford Department of Psychiatry (KJP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization, investigation, writing, review, and editing of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Itskovich, E., Bowling, D.L., Garner, J.P. et al. Oxytocin and the social facilitation of placebo effects. Mol Psychiatry 27, 2640–2649 (2022). https://doi.org/10.1038/s41380-022-01515-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01515-9
This article is cited by
-
The effect of oxytocin nasal spray on social interaction in young children with autism: a randomized clinical trial
Molecular Psychiatry (2023)
-
Biological principles for music and mental health
Translational Psychiatry (2023)