Key Points
-
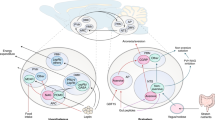
Recent findings indicate that neuronal populations in the hypothalamus that had already been identified as being crucial to the regulation of energy balance are also essential for the regulation of glucose homeostasis.
-
The melanocortin system within the arcuate nucleus (ARC) of the hypothalamus has an important role in the integration of signals from circulating hormones to maintain both energy and glucose homeostasis.
-
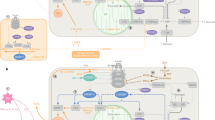
ATP-sensitive potassium channels, ATP-activated protein kinase and mammalian target of rapamycin act as 'general' fuel sensors — they sense changes in overall energy status through changes in ATP. The sensing of fuel and ATP specifically within the hypothalamus has the capacity to modulate both glucose and energy homeostasis.
-
Neuronal populations outside the ARC (including steroidogenic factor 1 neurons within the ventromedial hypothalamus) and multiple populations of neurons within the hindbrain (melanocortin receptor 4-expressing neurons in the sympathetic nervous system and NMDA receptor-expressing neurons) contribute to the regulation of glucose and energy homeostasis.
-
Within the gut, cholecystokinin and glucagon-like peptide 1 provide a conduit for CNS-induced regulation of energy and glucose homeostasis.
-
A key outstanding question in the field is whether the neuronal circuits that are crucial for body weight regulation and that may be dysregulated in obesity also contribute to the poor glucose homeostasis that eventually results in type 2 diabetes mellitus.
-
New strategies such as optogenetics and DREADD (designer receptors exclusively activated by designer drugs) provide an avenue for further understanding the crossroads of glucose and energy homeostasis.
Abstract
Obesity and type 2 diabetes mellitus (T2DM) — disorders of energy homeostasis and glucose homeostasis, respectively — are tightly linked and the incidences of both conditions are increasing in parallel. The CNS integrates information regarding peripheral nutrient and hormonal changes and processes this information to regulate energy homeostasis. Recent findings indicate that some of the neural circuits and mechanisms underlying energy balance are also essential for the regulation of glucose homeostasis. We propose that disruption of these overlapping pathways links the metabolic disturbances associated with obesity and T2DM. A better understanding of these converging mechanisms may lead to therapeutic strategies that target both T2DM and obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bernard, C. in Homeostasis: Origins of the Concept, 1973 (ed. Langley, L. L.) 129–151 (Dowden, Hutchinson & Ross, Stroudsberg, PA, 1870).
Knowler, W. C. et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374, 1677–1686 (2009).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Schwartz, M. W., Seeley, R. J., Campfield, L. A., Burn, P. & Baskin, D. G. Identification of hypothalmic targets of leptin action. J. Clin. Invest. 98, 1101–1106 (1996).
Hahn, T. M., Breininger, J. F., Baskin, D. G. & Schwartz, M. W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neurosci. 1, 271–272 (1998).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nature Neurosci. 8, 571–578 (2005). An excellent review on the various players in the melanocortin system and its role in energy balance.
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Woods, S. C., Seeley, R. J., Porte, D. J. & Schwartz, M. W. Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 (1998).
Brüning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Pelleymounter, M. A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995).
Baskin, D. G., Breininger, J. F. & Schwartz, M. W. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 48, 828–833 (1999).
Baskin, D. G. et al. Insulin and leptin: dual adipodity signals to the brain for the regulation of food intake and body weight. Brain Res. 848, 114–123 (1999).
Schwartz, M. W. et al. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Woods, S. C., Lotter, E. C., McKay, L. D. & Porte, D. Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282, 503–505 (1979).
Chavez, M., Kaiyala, K., Madden, L. J., Schwartz, M. W. & Woods, S. C. Intraventricular insulin and the level of maintained body weight in rats. Behav. Neurosci. 109, 528–531 (1995).
Seeley, R. J. et al. Intraventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Horm. Metab. Res. 28, 664–668 (1996).
Abate, N., Garg, A., Peshock, R. M., Stray-Gundersen, J. & Grundy, S. M. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Invest. 96, 88–98 (1995).
Woods, S. C., Seeley, R. J., Porte, D. Jr & Schwartz, M. W. Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 (1998).
Cota, D. et al. Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 (2006). An early demonstration that mTOR is a fuel sensor within the hypothalamus and regulates energy balance.
Clegg, D. J., Wortman, M. D., Benoit, S. C., McOsker, C. C. & Seeley, R. J. Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Diabetes 51, 3196–3201 (2002).
Loftus, T. M. et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288, 2299–2300 (2000).
Vaisse, C., Clement, K., B., G.-G. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genet. 20, 113–114 (1998).
Clegg, D. J. et al. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R981–R986 (2005).
Ryan, K. K., Woods, S. C. & Seeley, R. J. Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metab. 15, 137–149 (2012).
von Mering, J. & Minkowski, O. Diabetes Mellitus nach Pankreasextirpation. Zbl. Klin. Med. 10, 393–394 (1889).
Banting, F. G., Best, C. H., Collip, J. B., Campbell, W. R. & Fletcher, A. A. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 12, 141–146 (1922).
Macleod, J. J. Pancreatic extract and diabetes. Can. Med. Assoc. J. 12, 423–425 (1922).
Obici, S., Zhang, B. B., Karkanias, G. & Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nature Med. 8, 1376–1382 (2002).
Liu, L. et al. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J. Biol. Chem. 273, 31160–31167 (1998).
Obici, S., Feng, Z., Karkanias, G., Baskin, D. G. & Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nature Neurosci. 5, 566–572 (2002).
Benoit, S. C. et al. The catabolic action of insulin in the brain is mediated by melanocortins. J. Neurosci. 22, 9048–9052 (2002).
Konner, A. C. et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell. Metab. 5, 438–449 (2007). An excellent study that systematically activated IRs in AGRP- and/or POMC-expressing neurons to determine the necessity of these receptors for glucose homeostasis.
Lin, H. V. et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59, 337–346 (2010).
Pocai, A. et al. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54, 3182–3189 (2005).
Gutierrez-Juarez, R., Obici, S. & Rossetti, L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J. Biol. Chem. 279, 49704–49715 (2004).
German, J. et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150, 4502–4511 (2009).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
Shi, H. et al. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 294, E630–E639 (2008).
Berglund, E. D. et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Invest. 122, 1000–1009 (2012). An excellent study from the Elmquist laboratory that dissected the physiological role of leptin by manipulating OBRs in mouse models.
Hill, J. W. et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 11, 286–297 (2010).
Williams, K. W. et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 30, 2472–2479 (2010).
Levin, B. E. Metabolic sensing neurons and the control of energy homeostasis. Physiol. Behav. 89, 486–489 (2006).
Wang, R. et al. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J. Neurophysiol. 95, 1491–1498 (2006).
Lam, T. K., Gutierrez-Juarez, R., Pocai, A. & Rossetti, L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 309, 943–947 (2005).
Obici, S. et al. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51, 271–275 (2002).
Su, Y. et al. Hypothalamic leucine metabolism regulates liver glucose production. Diabetes 61, 85–93 (2012).
Parton, L. E. et al.Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449, 228–232 (2007).
Levin, B. E., Routh, V. H., Kang, L., Sanders, N. M. & Dunn-Meynell, A. A. Neuronal glucosensing: what do we know after 50 years? Diabetes 53, 2521–2528 (2004).
Pocai, A., Obici, S., Schwartz, G. J. & Rossetti, L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 1, 53–61 (2005). In this paper, the Rossetti group shows that central inhibition of fat oxidation is dependent on the activation of K ATP channels.
Pocai, A. et al. Hypothalamic KATP channels control hepatic glucose production. Nature 434, 1026–1031 (2005).
Kong, D. et al. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 12, 545–552 (2010).
Minokoshi, Y. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574 (2004).
Andersson, U. et al. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 279, 12005–12008 (2004).
Hardie, D. G. & Carling, D. The AMP-activated protein kinase — fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273 (1997).
Perrin, C., Knauf, C. & Burcelin, R. Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, controls muscle glycogen synthesis. Endocrinology 145, 4025–4033 (2004).
Claret, M. et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest. 117, 2325–2336 (2007).
Lindsley, J. E. & Rutter, J. Nutrient sensing and metabolic decisions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 139, 543–559 (2004).
Blouet, C., Ono, H. & Schwartz, G. J. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 8, 459–467 (2008).
Ono, H. et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J. Clin. Invest. 118, 2959–2968 (2008).
Ryan, K. K. et al. A role for central nervous system PPAR-[gamma] in the regulation of energy balance. Nature Med. 17, 623–626 (2011).
Derosa, G. & Maffioli, P. Effects of thiazolidinediones and sulfonylureas in patients with diabetes. Diabetes Technol. Ther. 12, 491–501 (2010).
Lu, M. et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nature Med. 17, 618–622 (2011).
Diano, S. et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nature Med. 17, 1121–1127 (2011). Interesting study showing a role for hypothalamic reactive oxygen species in the modulation of the melanocortin system.
De Fanti, B. A., Hamilton, J. S. & Horwitz, B. A. Meal-induced changes in extracellular 5-HT in medial hypothalamus of lean (Fa/Fa) and obese (fa/fa) Zucker rats. Brain Res. 902, 164–170 (2001).
Heisler, L. K. et al. Activation of central melanocortin pathways by fenfluramine. Science 297, 609–611 (2002). A seminal paper that demonstrated that the melanocortin neurocircuitry interacts with the 5-HT system to modulate energy balance.
Nonogaki, K., Strack, A. M., Dallman, M. F. & Tecott, L. H. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nature Med. 4, 1152–1156 (1998).
Zhou, L. et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 6, 398–405 (2007).
Ader, M. et al. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes 54, 862–871 (2005).
Kiss, J., Leranth, C. & Halasz, B. Serotoninergic endings on VIP-neurons in the suprachiasmatic nucleus and on ACTH-neurons in the arcuate nucleus of the rat hypothalamus. A combination of high resolution autoradiography and electron microscopic immunocytochemistry. Neurosci. Lett. 44, 119–124 (1984).
Xu, Y., Elmquist, J. K. & Fukuda, M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann. NY Acad. Sci. 1243, 1–14 (2011).
Xu, Y. et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60, 582–589 (2008).
Wade, J. M. et al. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology 149, 955–961 (2008).
Sohn, J. W. et al. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71, 488–497 (2011).
Qiu, J., Fang, Y., Ronnekleiv, O. K. & Kelly, M. J. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J. Neurosci. 30, 1560–1565 (2010).
Xu, Y. et al. A serotonin and melanocortin circuit mediates D-fenfluramine anorexia. J. Neurosci. 30, 14630–14634 (2010).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Obici, S. et al. Central melanocortin receptors regulate insulin action. J. Clin. Invest. 108, 1079–1085 (2001).
Hetherington, A. & Ranson, S. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 78, 149–172 (1940).
Rohner, F. et al. Immediate effect of lesion of the ventromedial hypothalamic area upon glucose-induced insulin secretion in anaesthetized rats. Diabetologia 13, 239–242 (1977).
Borg, W. P. et al. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J. Clin. Invest. 93, 1677–1682 (1994).
Klockener, T. et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nature Neurosci. 14, 911–918 (2011). One of many excellent papers from the Bruning laboratory, defining insulin action in SF1 and VMH neurons.
Dhillon, H. et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006).
Bingham, N. C., Anderson, K. K., Reuter, A. L., Stallings, N. R. & Parker, K. L. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149, 2138–2148 (2008).
Zhang, R. et al. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology 149, 5654–5661 (2008).
Tong, Q. et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 5, 383–393 (2007).
Grill, H. J. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity 14, 216S–221S (2006).
Grill, H. J., Ginsberg, A. B., Seeley, R. J. & Kaplan, J. M. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 18, 10128–10135 (1998).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011).
Cheung, G. W., Kokorovic, A., Lam, C. K., Chari, M. & Lam, T. K. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 10, 99–109 (2009).
Lam, T. K. et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nature Med. 11, 320–327 (2005).
DiRocco, R. J. & Grill, H. J. The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science 204, 1112–1114 (1979).
Hisadome, K., Reimann, F., Gribble, F. M. & Trapp, S. CCK stimulation of GLP-1 neurons involves α1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes 60, 2701–2709 (2011).
Rasmussen, B. A. et al. Duodenal activation of cAMP-dependent protein kinase induces vagal afferent firing and lowers glucose production in rats. Gastroenterology 142, 834–843.e3 (2012).
Strader, A. D. & Woods, S. C. Gastrointestinal hormones and food intake. Gastroenterology 128, 175–191 (2005).
Wang, P. Y. et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 452, 1012–1016 (2008).
Lam, C. K. et al. Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J. Biol. Chem. 285, 21913–21921 (2010).
Shimizu, I., Hirota, M., Ohboshi, C. & Shima, K. Identification and localization of glucagon-like peptide-1 and its receptor in the brain. Endocrinology 121, 1076–1082 (1987).
Barrera, J. G., Sandoval, D. A., D'Alessio, D. A. & Seeley, R. J. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nature Rev. Endocrinol. 7, 507–516 (2011).
Prigeon, R. L., Quddusi, S., Paty, B. & D'Alessio, D. A. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am. J. Physiol. Endocrinol. Metab. 285, E701–E707 (2003).
Sandoval, D. A., Bagnol, D., Woods, S. C., D'Alessio, D. A. & Seeley, R. J. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57, 2046–2054 (2008).
Cani, P. D. et al. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55, 1484–1490 (2006).
Kieffer, T. J. & Habener, J. F. The glucagon-like peptides. Endocr. Rev. 20, 876–913 (1999).
Vahl, T. P. et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148, 4965–4973 (2007).
Amato, A. et al. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol. Motil. 22, 664–e203 (2010).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neurosci. 14, 351–355 (2011). The first demonstration of the use of optogenetics in the field of energy balance. It used optogenetics to modulate AGRP.
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011). A ground-breaking study in the field of energy balance that demonstrated the power of DREADD technology.
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Moore, M. C. et al. Sources of carbon for hepatic glycogen synthesis in the conscious dog. J. Clin. Invest. 88, 578–587 (1991).
Xie, H. & Lautt, W. W. Insulin resistance caused by hepatic cholinergic interruption and reversed by acetylcholine administration. Am. J. Physiol. 271, E587–E592 (1996).
Wei, Y., Wang, D., Topczewski, F. & Pagliassotti, M. J. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J. Nutr. Biochem. 18, 1–9 (2007).
Bagger, J. I., Knop, F. K., Holst, J. J. & Vilsbøll, T. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes. Metab. 13, 965–971 (2011).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 33, S62–S69 (2010).
Acknowledgements
We are grateful for the helpful comments of S.C. Woods and S.C. Benoit. The work of the laboratory is supported in part by the US National Institutes of Health (NIH) Awards DK56863, DK57900, U01CA141464, DK082480, MH069860, DK082480 and also work with Ethicon Endo-Surgery, F. Hoffman-La Roche, Pfizer and Novo Nordisk A/S. B.E.G. is also supported by NIH Award 1F32HD68103.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.A.S. has received research support from Ethicon Endo-Surgery, Mannkind and Novo Nordisk. R.J.S. has received research support from Ethicon Endo-Surgery, Mannkind, Novo Nordisk, Pfizer and Roche. R.J.S. has served on scientific advisory boards for Ethicon Endo-Surgery, Angiochem and Novo Nordisk. R.J.S. is also a paid speaker for Merck, Ethicon Endo-Surgery, Pfizer and Novo Nordisk.
Related links
Glossary
- Glucose fluxes
-
Changes in glucose uptake, glucose production by the liver and glucose metabolism within a tissue or cell.
- Melanocortin system
-
Neurons in the arcuate nucleus of the hypothalamus and the nucleus of the solitary tract that express pro-opiomelanocortin, agouti-related protein and neuropeptide Y with downstream actions on melanocortin receptor 3 and melanocortin receptor 4.
- Antagonist/inverse agonist
-
A ligand that can both block a receptor's activity and produce the opposite action of the agonist.
- Gluconeogenesis
-
The process of making glucose from non-glucose precursors such as lactate, glycerol and alanine.
- Glycogenolysis
-
The breakdown of glycogen to glucose.
- Hepatic insulin sensitivity
-
The degree to which insulin suppresses glucose production by the liver.
- Glucose tolerance test
-
(GTT). A test that measures the glucose excursion after a bolus administration of glucose; it is an index of the body's ability to tolerate and/or handle a glucose load.
- Insulin tolerance tests
-
Tests that measure the fall in glucose levels after an injection of a bolus of insulin.
- Whole-body insulin sensitivity
-
The degree to which the body responds to insulin; it is usually assessed by comparing the glucose infusion rate during a hyperinsulinaemic–euglcyaemic clamp in control versus experimental animals.
- Mass action of glucose
-
Refers to glucose uptake into tissues, which is driven by how much glucose there is in the blood.
- Insulin resistance
-
A metabolic state in which the tissues of the body exhibit reduced responsiveness to chronically high levels of insulin in the circulation.
- Negative-feedback proteins
-
Proteins that suppress signalling by other molecules.
- Leptin resistance
-
A state in which the body is no longer responsive to the anorexic effect of exogenous leptin.
- Nodose ganglia
-
Inferior ganglia of the vagus nerve.
Rights and permissions
About this article
Cite this article
Grayson, B., Seeley, R. & Sandoval, D. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci 14, 24–37 (2013). https://doi.org/10.1038/nrn3409
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3409
This article is cited by
-
The ventromedial hypothalamic nucleus: watchdog of whole-body glucose homeostasis
Cell & Bioscience (2022)
-
Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure
Molecular Psychiatry (2022)
-
Mechanistic/mammalian target of rapamycin and side effects of antipsychotics: insights into mechanisms and implications for therapy
Translational Psychiatry (2022)
-
Circadian blueprint of metabolic pathways in the brain
Nature Reviews Neuroscience (2019)
-
Fructo-oligosaccharides and glucose homeostasis: a systematic review and meta-analysis in animal models
Nutrition & Metabolism (2018)