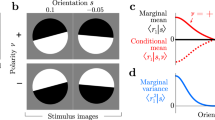
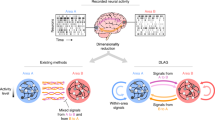
Key Points
-
To understand complex brain processes, there is a clear need to shift from traditional single-cell studies of trial-averaged responses to single-trial analyses of multiple neurons. In this respect, the decoding and information-theory formalisms offer a powerful framework to study how the brain computes information from the single-trial activity of neuronal populations.
-
Compared with single-cell studies, population analysis with decoding and information theory has several advantages: the information of the neuronal population is considered as a whole; information is extracted from single-trial occurrences; it is possible to discover which stimulus features are encoded by the neural responses; it is possible to evaluate which features of the neural responses carry relevant information; and it is possible to combine information from different types of neural signals.
-
Several studies have shown how much more knowledge can be extracted using the decoding and information-theory methodologies and how, in some cases, information that it is ambiguous at the single-cell level can be clearly interpreted when considering the whole population.
-
Decoding has the advantage of being similar to real behavioural calculations, but it may lose information contained in the neural responses. Information theory considers all the information in the neural response, but it is more difficult to compute for large populations and its values may not be biologically relevant.
-
The complementary knowledge offered by decoding and information theory has not been exploited enough in neuroscience. A joint application of both approaches may offer additional insights into how neuronal populations encode information.
Abstract
To a large extent, progress in neuroscience has been driven by the study of single-cell responses averaged over several repetitions of stimuli or behaviours. However,the brain typically makes decisions based on single events by evaluating the activity of large neuronal populations. Therefore, to further understand how the brain processes information, it is important to shift from a single-neuron, multiple-trial framework to multiple-neuron, single-trial methodologies. Two related approaches — decoding and information theory — can be used to extract single-trial information from the activity of neuronal populations. Such population analysis can give us more information about how neurons encode stimulus features than traditional single-cell studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hubel, D. Tungsten microelectrode for recording from single units. Science 125, 549–550 (1957).
Kandel, E. R., Schwartz, J. H. & Jessell, T. M. Principles of Neural Science (McGraw Hill, New York, 2000).
Schwartz, A. B. Cortical neural prosthetics. Annu. Rev. Neurosci. 27, 487–507 (2004).
Buzsaki, G. Large-scale recording of neuronal ensembles. Nature Neurosci. 7, 446–451 (2004).
Csicsvari, J. et al. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J. Neurophysiol. 90, 1314–1323 (2003).
Kelly, R. C. et al. Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. J. Neurosci. 27, 261–264 (2007).
Rousche, P. J. & Normann, R. A. Chronic recording capability of the Utah intracortical electrode in cat sensory cortex. J. Neurosci. Methods 82, 1–15 (1998).
Blanche, T. J., Spacek, M. A., Hetke, J. F. & Swindale, N. V. Polytrodes: high-density silicon electrode arrays for large-scale multiunit recording. J. Neurophysiol. 93, 2987–3000 (2005).
Brown, E. N., Kass, R. E. & Mitra, P. P. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nature Neurosci. 7, 456–461 (2004).
Abbott, L. F. Decoding neuronal firing and modelling neural networks. Q. Rev. Biophys. 27, 291–331 (1994).
Pouget, A., Dayan, P. & Zemel, R. Information processing with population codes. Nature Rev. Neurosci. 1, 125–132 (2000).
Rieke, F., Warland, D., de Ruyter van Steveninck, R. R. & Bialek, W. Spikes: Exploring the Neural Code (MIT Press, Cambridge, Massachusetts, 1997).
Oram, M. W., Foldiak, P., Perrett, D. I. & Sengpiel, F. The 'ideal homunculus': decoding neural population signals. Trends Neurosci. 21, 259–265 (1998).
Dayan, P. & Abbott, L. F. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems (MIT Press, Cambridge, Massachusetts, 2001).
Shannon, C. E. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 & 623–656 (1948).
Deco, G. & Obradovic, D. An Information-Theoretic Approach to Neural Computing (Springer, Berlin, 1997).
Borst, A. & Theunissen, F. E. Information theory and neural coding. Nature Neurosci. 2, 947–957 (1999).
Quian Quiroga, R. Spike sorting. Scholarpedia 2, 3583 (2007). A short review describing the steps for processing neural data, basically focused on spike detection and sorting.
Quian Quiroga, R., Nadasdy, Z. & Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 16, 1661–1687 (2004).
Lewicki, M. A review of methods for spike sorting: the detection and classification of neural action potentials. Network 9, R53–R78 (1998).
Harris, K. D., Henze, D. A., Csicsvari, J., Hirase, H. & Buzsaki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 84, 401–414 (2000).
Singer, W. & Gray, C. M. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 18, 555–586 (1995).
Engel, A. K. & Singer, W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn. Sci. 5, 16–25 (2001).
Quian Quiroga, R., Reddy, L., Kreiman, G., Koch, C. & Fried, I. Invariant visual representation by single neurons in the human brain. Nature 435, 1102–1107 (2005).
Reich, D. S., Mechler, F. & Victor, J. D. Independent and redundant information in nearby cortical neurons. Science 294, 2566–2568 (2001).
Barlow, H. B., Hill, R. M. & Levick, W. R. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J. Physiol. (Lond.) 173, 377–407 (1964).
MacKay, D. M. Information Theory, Inference, and Learning Algorithms (Cambridge Univ. Press, Cambridge, 2003).
Foldiak, P. in Computation and Neural Systems (eds Eeckman, F. H. & Bower, J.) 55–60 (Kluwer, Norwell, Massachusetts, 1993).
Sanger, T. D. Probability density estimation for the interpretation of neural population codes. J. Neurophysiol. 76, 2790–2793 (1996).
Paradiso, M. A. A theory for the use of visual orientation information which exploits the columnar structure of striate cortex. Biol. Cybern. 58, 35–49 (1988).
Duda, O. H., Hart, P. E. & Stork, D. G. Pattern Classification (Wiley & sons, New York, 2001).
Kjaer, T. W., Hertz, J. A. & Richmond, B. J. Decoding cortical neuronal signals: network models, information estimation and spatial tuning. J. Comp. Neurosci. 1, 109–139 (1994).
Averbeck, B. B. in Coherent Behavior in Neuronal Networks (ed. Rubin, J., Matias, M. and Romo, R.) (Springer, New York, in the press).
Cover, T. M. & Thomas, J. A. Elements of Information Theory. (Wiley & sons, Hoboken, New Jersey, 2006).
Victor, J. D. Approaches to information-theoretic analysis of neural activity. Biol. Theory 1, 302–316 (2006).
Panzeri, S., Senatore, R., Montemurro, M. A. & Petersen, R. S. Correcting for the sampling bias problem in spike train information measures. J. Neurophysiol. 98, 1064–1072 (2007).
Samengo, I. Information loss in an optimal maximum likelihood decoding. Neural Comput. 14, 771–779 (2002).
Treves, A. On the perceptual structure of face space. Biosystems 40, 189–196 (1997). One of the first papers to analyse the information given by the confusion matrix, showing that the distribution of incorrect stimulus predictions is important for understanding neural representations. This work was later extended to the study of how incorrect stimulus predictions relate to the mutual information between stimuli and responses in reference 47.
Panzeri, S., Treves, A., Schultz, S. & Rolls, E. T. On decoding the responses of a population of neurons from short time windows. Neural Comput. 11, 1553–1577 (1999).
Robertson, R. G., Rolls, E. T., Georges-Francois, P. & Panzeri, S. Head direction cells in the primate pre-subiculum. Hippocampus 9, 206–219 (1999).
Georgopoulos, A. P., Schwartz, A. B. & Kettner, R. E. Neuronal population coding of movement direction. Science 233, 1416–1419 (1986). The first study to implement a population analysis of neuronal responses by using a population vector.
Zhang, K., Ginzburg, I., McNaughton, B. L. & Sejnowski, T. J. Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J. Neurophysiol. 79, 1017–1044 (1998).
Knill, D. C. & Pouget, A. The bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 27, 712–719 (2004).
Quian Quiroga, R., Reddy, L., Koch, C. & Fried, I. Decoding visual inputs from multiple neurons in the human temporal lobe. J. Neurophysiol. 98, 1997–2007 (2007). This paper showed that it is possible to correctly predict picture presentations from the firing of neurons in the human medial temporal lobe far above chance. The authors also showed that more information can be extracted from a population analysis than from a single-cell study.
Victor, J. D. & Purpura, K. P. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J. Neurophysiol. 76, 1310–1326 (1996).
Schnupp, J. W. H., Hall, T. M., Kokelaar, R. F. & Ahmed, B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J. Neurosci. 26, 4785–4795 (2006).
Thomson, E. E. & Kristan, W. B. Quantifying stimulus discriminability: a comparison of information theory and ideal observer analysis. Neural Comput. 17, 741–778 (2005).
Ma, W. J., Beck, J. M., Latham, P. E. & Pouget, A. Bayesian inference with probabilistic population codes. Nature Neurosci. 9, 1432–1438 (2006).
Beck, J. M. et al. Probabilistic population codes for Bayesian decision making. Neuron 60, 1142–1152 (2008).
Averbeck, B. B., Sohn, J.-W. & Lee, D. Activity in prefrontal cortex during dynamic selection of action sequences. Nature Neurosci. 9, 276–282 (2006).
Pouget, A., Zhang, K., Deneve, S. & Latham, P. E. Statistically efficient estimation using population coding. Neural Comput. 10, 373–401 (1998).
Sahani, M. & Dayan, P. Doubly distributional population codes: simultaneous representation of uncertainty and multiplicity. Neural Comput. 15, 2255–2279 (2003).
Pouget, A., Zemel, R. & Dayan, P. Inference and computation with population code. Annu. Rev. Neurosci. 26, 381–410 (2003).
Victor, J. D. & Nirenberg, S. Indices for testing neural codes. Neural Comput. 20, 2895–2936 (2008). The authors analysed in detail the relative strengths and weaknesses of information theory and Bayesian decoders when they are used to rule out neural codes. They also introduced a set of measures that varied smoothly between information theory and Bayesian decoders.
Andersen, R. A. & Buneo, C. A. Intentional maps in the posterior parietal cortex. Annu. Rev. Neurosci. 25, 189–220 (2002).
Mountcastle, V. B., Lynch, J. C., Georgopoulos, A., Sakata, H. & Acuna, C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophysiol. 38, 871–908 (1975).
Andersen, R. A., Essick, G. K. & Siegel, R. M. Neurons of area 7 activated by both visual stimuli and oculomotor behavior. Exp. Brain Res. 67, 316–322 (1987).
Snyder, L. H., Batista, A. P. & Andersen, R. A. Coding of intention in the posterior parietal cortex. Nature 386, 167–170 (1997).
Bisley, J. W. & Goldberg, M. E. Neuronal activity in the lateral intraparietal area and spatial attention. Science 299, 81–81 (2003).
Robinson, D. L., Goldberg, M. E. & Stanton, G. B. Parietal association cortex in the primate: sensory mechanisms and behavioral modulations. J. Neurophysiol. 41, 910–932 (1978).
Quian Quiroga, R., Snyder, L. H., Batista, A. P., Cui, H. & Andersen, R. A. Movement intention is better predicted than attention in the posterior parietal cortex. J. Neurosci. 26, 3615–3620 (2006). Using a decoding population analysis, the authors showed that neurons in the posterior parietal cortex encode different movement plans and not just attention to target location.
Musallam, S., Corneil, B., Greger, B., Scherberger, H. & Andersen, R. A. Cognitive control signals for neural prosthetics. Science 305, 258–262 (2004).
Pesaran, B., Pezaris, J., Sahani, M., Mitra, P. M. & Andersen, R. A. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nature Neurosci. 5, 805–811 (2002).
Scherberger, H., Jarvis, M. & Andersen, R. A. Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron 46, 347–354 (2005).
Wessberg, J. et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature 408, 361–365 (2000).
Andersen, R. A., Burdick, J. W., Musallam, S., Pesaran, B. & Cham, J. G. Cognitive neural prosthetics. Trends Cogn. Sci. 8, 486–493 (2004).
Nicolelis, M. A. Actions from thoughts. Nature 409, 403–407 (2001).
Stopfer, M., Jayaraman, V. & Laurent, G. Intensity versus identity coding in an olfactory system. Neuron 39, 991–1004 (2003).
Arabzadeh, E., Panzeri, S. & Diamond, M. E. Whisker vibration information carried by rat barrel cortex neurons. J. Neurosci. 24, 6011–6020 (2004).
Adrian, E. D. The Basis of Sensations (Norton, New York, 1928).
Optican, L. M. & Richmond, B. J. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. III. Information theoretic analysis. J. Neurophysiol. 57, 162–178 (1987). The first study to demonstrate that the time profile of cortical spike trains encodes much more information about static visual features than spike counts.
de Ruyter van Steveninck, R. R., Lewen, G. D., Strong, S. P. & Koberle, R. Reproducibility and variability in neural spike trains. Science 275, 1805–1808 (1997).
Hopfield, J. J. Pattern recognition computation using action potential timing for stimulus representation. Nature 376, 33–36 (1995).
Theunissen, F. & Miller, J. P. Temporal encoding in nervous systems: a rigorous definition. J. Comp. Neurosci. 2, 149–162 (1995).
Victor, J. D. Temporal aspects of neural coding in the retina and lateral geniculate. Network 10, R1–R66 (1999).
Panzeri, S., Petersen, R. S., Schultz, S. R., Lebedev, M. & Diamond, M. E. The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron 29, 769–777 (2001).
Reinagel, P. & Clay Reid, R. Temporal coding of visual information in the thalamus. J. Neurosci. 20, 5392–5400 (2000).
Berry, M. J., Warland, D. K. & Meister, M. The structure and precision of retinal spike trains. Proc. Natl Acad. Sci. USA 94, 5411–5416 (1997).
Engineer, C. T. et al. Cortical activity patterns predict speech discrimination ability. Nature Neurosci. 11, 603–608 (2008).
Shadlen, M. N. & Movshon, J. A. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24, 67–77; 111–125 (1999).
Huxter, J. R., Senior, T. J., Allen, K. & Csicsvari, J. Theta phase–specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nature Neurosci. 11, 587–594 (2008). The authors recorded spiking activity and local field oscillations in the hippocampus of rats moving through a two-dimensional environment. Using a decoding analysis, they demonstrated that spikes emitted at different oscillation phases represent complementary aspects of the animal's trajectory.
Huxter, J. R., Burgess, N. & O'Keefe, J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature 425, 828–832 (2003).
Buracas, G. T., Zador, A., DeWeese, M. & Albright, T. Efficient encoding of rapidly varying stimuli by motion-sensitive neurons in MT of alert monkeys. Neuron 20, 959–969 (1998).
Kara, P., Reinagel, P. & Clay Reid, R. Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron 27, 635–646 (2000).
Theunissen, F. E. From synchrony to sparseness. Trends Neurosci. 26, 61–64 (2003).
Perez-Orive, J. et al. Oscillations and sparsening of odor representations in the mushroom body. Science 297, 359–365 (2002).
Hahnloser, R. H. R., Kozhevnikov, A. A. & Fee, M. S. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419, 65–70 (2002).
Salinas, E., Hernandez, A., Zainos, A. & Romo, R. Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J. Neurosci. 20, 5503–5515 (2000).
Arabzadeh, E., Panzeri, S. & Diamond, M. E. Deciphering the spike train of a sensory neuron: counts and temporal patterns in the rat whisker pathway. J. Neurosci. 26, 9216–9226 (2006). This paper developed an analytical formalism that quantifies how much sensory information can be extracted from the spike times when the stimulus time is not known precisely.
Reich, D. S., Mechler, F. & Victor, J. D. Temporal coding of contrast in primary visual cortex: when, what, and why. J. Neurophysiol. 85, 1039–1050 (2001).
Gawne, T. J., Kjaer, T. W. & Richmond, B. J. Latency: another potential code for feature binding in striate cortex. J. Neurophysiol. 76, 1356–1360 (1996).
Muller, J. R., Metha, A. B., Krauskopf, J. & Lennie, P. Information conveyed by onset transients in responses of striate cortical neurons. J. Neurosci. 21, 6978–6990 (2001).
Ahrens, K. F. & Kleinfeld, D. Current flow in vibrissa motor cortex can phase-lock with exploratory rhythmic whisking in rat. J. Neurophysiol. 92, 1700–1707 (2004).
Abbott, L. F. & Dayan, P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101 (1999).
Averbeck, B. B., Latham, P. E. & Pouget, A. Neural correlations, population coding and computation. Nature Rev. Neurosci. 7, 358–366 (2006). An up-to-date and comprehensive review of the role of noise correlations in population coding.
Nirenberg, S. & Latham, P. E. Decoding neuronal spike trains: how important are correlations? Proc. Natl Acad. Sci. USA 100, 7348–7353 (2003).
Salinas, E. & Sejnowski, T. J. Correlated neuronal activity and the flow of neural information. Nature Rev. Neurosci. 2, 539–550 (2001).
Zohary, E., Shadlen, M. & Newsome, W. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370, 140–143 (1994).
Panzeri, S., Schultz, S., Treves, A. & Rolls, E. T. Correlations and the encoding of information in the nervous system. Proc. R. Soc. Lond. B Biol. Sci. 266, 1001–1012 (1999).
Petersen, R. S., Panzeri, S. & Diamond, M. E. Population coding of stimulus location in rat somatosensory cortex. Neuron 32, 503–514 (2001).
Montani, F., Kohn, A., Smith, M. A. & Schultz, S. R. The role of correlations in direction and contrast coding in the primary visual cortex. J. Neurosci. 27, 2338–2348 (2007).
Nirenberg, S., Carcieri, S. M., Jacobs, A. L. & Latham, P. E. Retinal ganglion cells act largely as independent encoders. Nature 411, 698–701 (2001).
Dan, Y., Alonso, J. M., Usrey, W. M. & Clay Reid, R. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nature Neurosci. 1, 501–507 (1998).
Mitzdorf, U. Properties of the evoked potential generators: current source-density analysis of visually evoked potentials in the cat cortex. Int. J. Neurosci. 33, 33–59 (1987).
Juergens, E., Guettler, A. & Eckhorn, R. Visual stimulation elicits locked and induced gamma oscillations in monkey intracortical and EEG-potentials, but not in human EEG. Exp. Brain Res. 129, 247–259 (1999).
Harada, Y. & Takahashi, T. The calcium component of the action potential in spinal motoneurones of the rat. J. Physiol. 335, 89–100 (1983).
Kamondi, A., Acsady, L., Wang, X. J. & Buzsaki, G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus 8, 244–261 (1998).
Montemurro, M., Rasch, M. J., Murayama, Y., Logothetis, N. K. & Panzeri, S. Phase-of-firing coding of natural visual stimuli in primary visual cortex. Curr. Biol. 18, 375–380 (2008). This study used information theory to demonstrate that, in the macaque primary visual cortex, the phase of slow (<12 Hz) local field fluctuations encodes information about natural visual scenes that cannot be obtained from spike counts.
Belitski, A. et al. Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information. J. Neurosci. 28, 5696–5709 (2008).
Mehta, M. R., Lee, A. K. & Wilson, M. A. Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417, 741–746 (2002).
Harris, K. D. et al. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature 417, 738–741 (2002).
Harris, K. D. Neural signatures of cell assembly organization. Nature Rev. Neurosci. 6, 399–407 (2005).
Kraskov, A., Quian Quiroga, R., Reddy, L., Fried, I. & Koch, C. Local field potentials and spikes in the human medial temporal lobe are selective to image category. J. Cogn. Neurosci. 19, 479–492 (2007).
Kreiman, G. et al. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron 49, 433–445 (2006).
Andersen, R. A., Musallam, S. & Pesaran, B. Selecting the signals for a brain-machine interface. Curr. Opin. Neurobiol. 14, 720–726 (2004).
Schneiman, E., Berry, M. J., Segev, R. & Bialek, W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440, 1007–1012 (2006).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Haxby, J. V. et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293, 2425–2430 (2001).
Haynes, J.-D. & Rees, G. Decoding mental states from brain activity in humans. Nature Rev. Neurosci. 7, 523–534 (2006).
Eichele, T. et al. Assessing the spatiotemporal evolution of neuronal activation with single-trial event-related potentials and functional MRI. Proc. Natl Acad. Sci. USA 102, 17798–17803 (2005). First study using a single-trial analysis to combine the temporal and spatial resolutions of electroencephalogram and functional MRI recordings.
Debener, S., Ullsperger, M., Siegel, M. & Engel, A. K. Single-trial EEG-fMRI reveals the dynamics of cognitive function. Trends Cogn. Sci. 10, 558–563 (2006).
Gerstein, G. L. & Clark, W. A. Simultaneous studies of firing patterns in several neurons. Science 143, 1325–1327 (1964).
Vapnik, R. J. Statistical Learning Theory (Wiley & sons, New York, 1998).
Soong, T. T. Fundamentals of Probability and Statistics for Engineers (Wiley, Sussex, 2004).
Latham, P. E. & Nirenberg, S. Synergy, redundancy, and independence in population codes, revisited. J. Neurosci. 25, 5195–5206 (2005). This study derived a rigorous measure of the information that is lost when using algorithms that make incorrect assumptions about the probabilities of neural responses to stimuli. It provides a useful tool for understanding which features of the stimulus–response relationship are important for information transmission.
Acknowledgements
We are very thankful to F. Montani, K. Whittingstall, J. Csicsvari, A. Mazzoni and G. Kreiman for comments, to P. Dayan for interesting discussions about uncertainty and decoding, and to all our brilliant colleagues that collaborated with us on these topics: R. Andersen, M. Diamond, I. Fried, C. Koch, N. Logothetis, C. Kayser, M. Montemurro, R. Petersen and A. Treves. We acknowledge support from the Engineering and Physical Sciences Research Council, the Medical Research Council, the Royal Society and the Italian Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Spike sorting
-
The grouping of spikes into clusters based on the similarity of their shapes. Given that, in principle, each neuron tends to fire spikes of a particular shape, the resulting clusters correspond to the activity of different putative neurons. The end result of spike sorting is determining which spike corresponds to which of these neurons.
- Decoding
-
Predicting the most likely stimulus or behaviour eliciting an observed neural response.
- Information theory
-
A mathematical theory that deals with measures of information and their application to the study of communication systems. In neuroscience it is used to establish the amount of information about a stimulus or behaviour that is contained in the neural responses.
- Local field potential
-
(LFP). A neurophysiological signal that is obtained by low-pass filtering extracellular recordings. It represents the mean field potential generated by the slow components of synaptic and neural events in the vicinity of the recording electrode.
- Posterior probability
-
The posterior probability of a random variable is the conditional probability assigned to the variable given some event. For example, the posterior probability P(s|r) is the conditional probability that stimulus s was presented, given that a response r was observed.
- Shannon entropy
-
A measure of the uncertainty about the value that might be taken by a random variable.
- Bit
-
The unit used to measure reduction of uncertainty. One bit corresponds to a reduction of uncertainty by a factor of two (for example, a correct answer to a yes/no question).
- Unbiased decoder
-
A decoder is said to be unbiased if the expected value of its decoding error (the difference between the true and the estimated stimulus values) is zero.
- Brain–machine interface
-
A direct communication link between a brain (human or animal) and an external device, such as a prosthetic limb or a sensing device.
- Principal-component analysis
-
A linear transformation that projects the data on to an orthogonal base, in which the greatest variance of the data lies on the first coordinate (the first principal component), the second greatest variance on the second coordinate, and so on. It is usually used to reduce the dimensionality of complex data.
- Spike afterpotential
-
A transient hyperpolarization of a neuron following the firing of an action potential. It is caused by K+ channels, which open during the spike and close a few milliseconds after the neural membrane potential goes back to its resting value.
- Voxel
-
In MRI research, a voxel refers to the smallest measured volume unit, analogous to a three-dimensional pixel. In functional MRI studies these are typically of the order of 30 mm3, although much smaller voxel volumes have been achieved in more recent work.
Rights and permissions
About this article
Cite this article
Quian Quiroga, R., Panzeri, S. Extracting information from neuronal populations: information theory and decoding approaches. Nat Rev Neurosci 10, 173–185 (2009). https://doi.org/10.1038/nrn2578
Issue Date:
DOI: https://doi.org/10.1038/nrn2578