Key Points
-
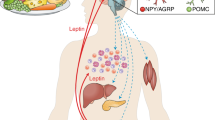
The body possesses an energy homeostasis system by which it adjusts food intake to match calories burned to keep body weight stable
-
The hormone leptin, which is made by adipose tissue in approximate proportion to fat stores, plays an important role in the control of energy homeostasis. When fat stores are expended, leptin falls, causing an increase in appetite and diminishing energy expenditure to return fat stores to their previous levels
-
Because obesity results from the accretion of adipose tissue fat stores, leptin levels are high in obesity. The failure of this high leptin (and therapy with exogenous leptin) in the obese state to decrease feeding and return adipose mass to normal has suggested the existence of leptin resistance, in which obesity impairs leptin action
-
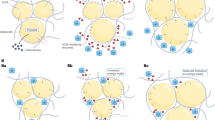
Obesity provokes a number of changes to hypothalamic anatomy and physiology, many of which have been invoked as potential mediators of leptin resistance. These include the leptin-induced expression of leptin signalling inhibitors, hypothalamic inflammatory signalling and gliosis and endoplasmic reticulum stress
-
A variety of data suggest that early steps in leptin signalling are appropriately enhanced in response to the high leptin levels in obesity and that elevated leptin itself may attenuate downstream leptin action. Hence, the failure of leptin to decrease food intake and body weight in obesity may result from high leptin levels producing changes that act downstream of the initial steps in leptin signalling to create a functional ceiling for leptin action
-
The available data suggest that leptin functions primarily in defence against decreased body weight rather than in limiting increases in body weight
Abstract
Obesity represents the single most important risk factor for early disability and death in developed societies, and the incidence of obesity remains at staggering levels. CNS systems that modulate energy intake and expenditure in response to changes in body energy stores serve to maintain constant body adiposity; the adipocyte-derived hormone leptin and its receptor (LEPR) represent crucial regulators of these systems. As in the case of insulin resistance, a variety of mechanisms (including feedback inhibition, inflammation, gliosis and endoplasmic reticulum stress) have been proposed to interfere with leptin action and impede the systems that control body energy homeostasis to promote or maintain obesity, although the relative importance and contribution of each of these remain unclear. However, LEPR signalling may be increased (rather than impaired) in common obesity, suggesting that any obesity-associated defects in leptin action must result from lesions somewhere other than the initial LEPR signal. It is also possible that increased LEPR signalling could mediate some of the obesity-associated changes in hypothalamic function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cutler, D. M., Glaeser, E. L. & Shapiro, J. M. Why have Americans become more obese? J. Econ. Perspect. 17, 93–118 (2003).
Cawley, J. & Meyerhoefer, C. The medical care costs of obesity: an instrumental variables approach. J. Health Econ. 31, 219–230 (2012).
Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Rosenbaum, M. et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 115, 3579–3586 (2005).
Ingalls, A. M., Dickie, M. M. & Snell, G. D. Obese, a new mutation in the house mouse. J. Hered. 41, 317–318 (1950).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This article describes the original cloning of leptin, showing that it is a hormone that is made by white adipose tissue and that it can decrease food intake in normal and leptin-deficient mice.
Frederich, R. C. et al. Leptin levels reflect body lipid-content in mice — evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Farooqi, S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Chua, S. C. Jr et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the ob (leptin) receptor. Science 271, 994–996 (2016).
Flak, J. N. & Myers, M. G. Minireview: CNS mechanisms of leptin action. Mol Endocrinol. 30, 3–12 (2016).
De Luca, C. et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 115, 3484–3493 (2005).
Lou, P.-H. et al. Reduced body weight and increased energy expenditure in transgenic mice over-expressing soluble leptin receptor. PLoS ONE 5, e11669 (2010).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996). This study treated fasted mice with leptin, reversing many components of the neuroendocrine starvation response (hypercortisolism, hypothyroidism, decreased energy expenditure and infertility). Thus, the decrease in leptin that accompanies calorific restriction contributes to the activation of the neuroendocrine starvation response.
Allison, M. B. & Myers, M. G. Connecting leptin signaling to biological function. J. Endocrinol. 223, T25–T35 (2014).
Piper, M. L., Unger, E. K., Myers, M. G. & Xu, A. W. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol. Endocrinol. 22, 751–759 (2008).
Bates, S. H. et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003).
Jiang, L. et al. Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc. Natl Acad. Sci. USA 105, 18619–18624 (2008).
Niswender, K. D. et al. Intracellular signalling: key enzyme in leptin-induced anorexia. Nature 413, 794–795 (2001).
Wang, Y. et al. Role of astrocytes in leptin signaling. J. Mol. Neurosci. 56, 829–839 (2015).
Allison, M. B. et al. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol. Metab. 4, 299–309 (2015).
Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017).
Waterson, M. J. & Horvath, T. L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 22, 962–970 (2015).
Kim, J. G. et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 17, 908–910 (2014).
Van De Wall, E. et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149, 1773–1785 (2008).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011). This study shows that leptin acts via non-AGRP-expressing inhibitory neurons to indirectly control the activity of POMC cells, providing the first evidence of important indirect regulation of POMC neurons by leptin.
Garfield, A. S. et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 19, 1628–1635 (2016). This study shows that GABAergic LEPR neurons in the DMH innervate AGRP cells to control their activity, suggesting that an important component of the regulation of AGRP neurons by leptin is indirect.
Leshan, R. L., Greenwald-Yarnell, M., Patterson, C. M., Gonzalez, I. E. & Myers, M. G. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat. Med. 18, 820–823 (2012). This study shows that non-POMC LEPR neurons that express Nos1 control gene expression in POMC cells, revealing that leptin controls many aspects of POMC neuron function indirectly.
Rezai-Zadeh, K. et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 3, 681–693 (2014).
Dodd, G. T. et al. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab. 20, 639–649 (2014).
Dhillon, H. et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006).
Hawke, Z. et al. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J. Neurosci. 29, 14828–14835 (2009).
Leinninger, G. M. et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323 (2011).
Laque, A. et al. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol. Metab. 4, 706–717 (2015).
Liu, J., Perez, S. M., Zhang, W., Lodge, D. J. & Lu, X.-Y. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol. Psychiatry 16, 1024–1038 (2011).
Goforth, P. B., Leinninger, G. M., Patterson, C. M., Satin, L. S. & Myers, M. G. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J. Neurosci. 34, 11405–11415 (2014).
Huo, L., Maeng, L., Bjørbæk, C. & Grill, H. J. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148, 2189–2197 (2007).
Morton, G. J. et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J. Clin. Invest. 115, 703–710 (2005).
Williams, D. L., Baskin, D. G. & Schwartz, M. W. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55, 3387–3393 (2006).
Grill, H. J. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143, 239–246 (2002).
Hayes, M. R. et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 11, 77–83 (2010).
Scott, M. M. et al. Leptin targets in the mouse brain. J. Comp. Neurol. 514, 518–532 (2009).
Patterson, C. M., Leshan, R. L., Jones, J. C. & Myers, M. G. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 1378, 18–28 (2011).
Yadav, V. K. et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138, 976–989 (2009).
Lam, D. D. et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 13, 584–591 (2011).
Flak, J. N. et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat. Neurosci. 17, 1744–1750 (2014).
Flak, J. N. et al. A leptin-regulated circuit controls glucose mobilization during noxious stimuli. J. Clin. Invest. 127, 3103–3113 (2017).
Kanoski, S. E. & Grill, H. J. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 81, 748–756 (2017).
El-Haschimi, K., Pierroz, D. D., Hileman, S. M., Bjørbæk, C. & Flier, J. S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 105, 1827–1832 (2000).
Jameson, J. Hormone Resistance Syndromes. (Springer Science & Business Media, 1999).
Münzberg, H., Flier, J. S. & Bjørbæk, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145, 4880–4889 (2004).
Bjørbaek, C., Elmquist, J. K., Frantz, J. D., Shoelson, S. E. & Flier, J. S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1, 619–625 (1998).
Bjørbæk, C. et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 276, 4747–4755 (2001).
Bjørbæk, C., El-Haschimi, K., Frantz, J. D. & Flier, J. S. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 274, 30059–30065 (1999).
Bjornholm, M. et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J. Clin. Invest. 117, 1354–1360 (2007).
Mori, H. et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 10, 739–743 (2004).
Zabolotny, J. M. et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2, 489–495 (2002).
White, C. L. et al. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. AJP Endocrinol. Metab. 296, E291–E299 (2008).
Bence, K. K. et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 12, 917–924 (2006).
Loh, K. et al. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab. 14, 684–699 (2011).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Thaler, J. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153 (2011). This study shows that obesity is associated with increased hypothalamic astrocyte number and activation in humans as well as in rodent models.
Zhang, X. et al. Hypothalamic IKKb/NF-kB ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Benoit, S. C. et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J. Clin. Invest. 119, 2577–2589 (2009).
Romanatto, T. et al. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient — effects on leptin and insulin signaling pathways. Peptides 28, 1050–1058 (2007).
Arruda, A. P. et al. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology 152, 1314–1326 (2011).
Sabio, G. et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 24, 256–264 (2010).
Tsaousidou, E. et al. Distinct roles for JNK and IKK activation in agouti-related peptide neurons in the development of obesity and insulin resistance. Cell Rep. 9, 1495–1506 (2014).
Benzler, J. et al. Central inhibition of IKKb/NF-kB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes 64, 2015–2027 (2015).
Milanski, M. et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 29, 359–370 (2009).
Butti, E. et al. Absence of an intrathecal immune reaction to a helper-dependent adenoviral vector delivered into the cerebrospinal fluid of non-human primates. Gene Ther. 15, 233–238 (2008).
Ropelle, E. R. et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biol. 8, e1000465 (2010).
Dong, Z. M., Gutierrez-Ramos, J. C., Coxon, A, Mayadas, T. N. & Wagner, D. D. A new class of obesity genes encodes leukocyte adhesion receptors. Proc. Natl Acad. Sci. USA 94, 7526–7530 (1997).
Langhans, W. Signals generating anorexia during acute illness. Proc. Nutr. Soc. 66, 321–330 (2007).
Valdearcos, M. et al. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 9, 2124–2139 (2014).
Dorfman, M. D. et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 8, 14556 (2017).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Allen, N. J. Astrocyte regulation of synaptic behavior. Annu. Rev. Cell Dev. Biol. 30, 439–463 (2014).
Ye, J. & McGuinness, O. P. Inflammation during obesity is not all bad: evidence from animal and human studies. AJP Endocrinol. Metab. 304, E466–E477 (2013).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009). This study shows that obesity is associated with the activation of pathways associated with ER stress in the hypothalamus and that increased ER stress can blunt leptin signalling.
Back, S. H. & Kaufman, R. J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 81, 767–793 (2012).
Marciniak, S. J. & Ron, D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 86, 1133–1149 (2006).
Williams, K. W. et al. Xbp1s in pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 20, 471–482 (2014).
Liu, J., Lee, J., Hernandez, M. A. S., Mazitschek, R. & Ozcan, U. Treatment of obesity with celastrol. Cell 161, 999–1011 (2015).
Lee, J. et al. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 22, 1023–1032 (2016).
Ottaway, N. et al. Diet-induced obese mice retain endogenous leptin action. Cell Metab. 21, 877–882 (2015).
Knight, Z. A., Hannan, K. S., Greenberg, M. L. & Friedman, J. M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 5, e11376 (2010). This study uses mice with 'clamped' leptin levels to show that obese mice that do not have high leptin levels remain sensitive to exogenous leptin. Thus, high leptin, rather than other processes associated with obesity, represents the major factor limiting leptin action in the obese state.
Flier, J. S. & Maratos-Flier, E. Leptin's physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 26, 24–26 (2017).
Müller, T. D. et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J. Pept. Sci. 18, 383–393 (2012).
Roth, J. D. et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc. Natl Acad. Sci. USA 105, 7257–7262 (2008).
Burke, L. K. & Heisler, L. K. 5-hydroxytryptamine medications for the treatment of obesity. J. Neuroendocrinol. 27, 389–398 (2015).
Solinas, G. et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 (2007).
Nguyen, M. T. A. et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282, 35279–35292 (2007).
Saberi, M. et al. Hematopoietic cell-specific deletion of Toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Wang, H. et al. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab. 13, 105–113 (2011).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Reijnders, D. et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab. 24, 63–74 (2016).
Simonds, S. E. et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416 (2014).
Lord, G. M. et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901 (1998).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 8, 519–529 (2007).
Henry, F. E., Sugino, K., Tozer, A., Branco, T. & Sternson, S. M. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 4, e09800 (2015).
Acknowledgements
The authors were supported by the Michigan Diabetes Research Center (P30 DK020572), the American Diabetes Association, the Marilyn H. Vincent Foundation, the US National Institutes of Health (DK56731 and DK78056) and the Cell and Molecular Biology (CMB) Training Grant (T32GM007315). The authors thank D. Olson, D. Sandoval, R. Seeley and members of the Myers laboratory for helpful discussions.
Author information
Authors and Affiliations
Contributions
M.G.M. was involved in researching data for the article, made a substantial contribution to discussion of content and wrote, reviewed and edited the manuscript before submission. W.W.P. was involved in researching data for the article, made a substantial contribution to the discussion of content and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Energy expenditure
-
The burning of calories by an organism on normal metabolism (basal metabolic rate) and activity.
- Hyperphagia
-
Literally meaning 'eating too much', it is the consumption of more calories than needed to maintain energy homeostasis and results in the deposition of excess calories in adipose tissue.
- Energy homeostasis
-
The process by which the number of calories eaten are matched to the number of calories burned to maintain a constant body weight; also known as energy balance.
- White adipose tissue
-
The tissue commonly thought of as fat; major depots are found under the skin and inside the abdominal cavity.
- Orexigenic
-
A type of stimuli that increases feeding.
- Systemic inflammation
-
An immune response to infection or other insults that increases the activity of immune cells in the body.
- Gut microbiome
-
The bacteria and other microorganisms that colonize the lumen of the gut.
Rights and permissions
About this article
Cite this article
Pan, W., Myers, M. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci 19, 95–105 (2018). https://doi.org/10.1038/nrn.2017.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.168
This article is cited by
-
Therapeutic uses of oxytocin in stress-related neuropsychiatric disorders
Cell & Bioscience (2023)
-
Association of a high-fat diet with I-FABP as a biomarker of intestinal barrier dysfunction driven by metabolic changes in Wistar rats
Lipids in Health and Disease (2023)
-
The melanocortin action is biased toward protection from weight loss in mice
Nature Communications (2023)
-
Beneficial Short-Term Effects of Bariatric Surgery on Nutritional Inflammatory Profile and Metabolic Biomarkers
Obesity Surgery (2023)
-
Refining the diagnosis of gestational diabetes mellitus: a systematic review and meta-analysis
Communications Medicine (2023)