Key Points
-
Electron cryotomography (ECT) can reveal the structure of intact cells in a native state in 3D to macromolecular (∼4 nm) resolution.
-
ECT bridges the resolution gap between atomic structures of individual proteins (solved by X-ray crystallography, NMR spectroscopy and cryo-electron microscopy single-particle reconstruction) and overall cell morphology as visualized by light microscopy.
-
ECT has provided new insights into many aspects of prokaryotic cell biology, including basic membrane structure, cell wall architecture, morphogenesis, basic metabolism, motility, chemotaxis, sporulation, inter-species cooperation and competition, and viral infection.
-
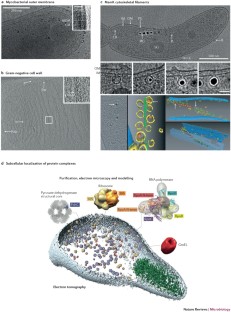
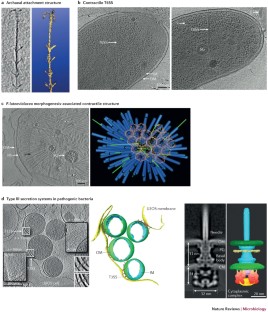
ECT has enabled the direct visualization, for the first time, of the arrangement of polymers in the bacterial cell wall. This revealed unexpected structural conservation between Gram-positive and Gram-negative envelopes, eroding the distinction between them, as well as conversion of an inner to an outer membrane during sporulation, which suggests a new hypothesis for the evolution of the diderm cell plan.
-
ECT has confirmed the existence of a bacterial cytoskeleton, and also uncovered its complexity, including diverse filaments mediating processes that range from shape maintenance to motility to division.
-
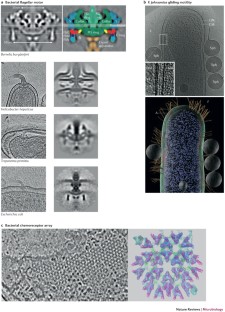
ECT has revealed the structures of large nanomachines (macromolecular complexes that comprise many unique components), such as flagellar motors, chemoreceptor arrays and secretion systems intact in their native state within cells. Because these nanomachines typically cannot be purified or reconstituted completely for traditional structural analysis, ECT has provided a necessary new source of information on their overall architecture. Placing atomic models of components into their relative positions within ECT reconstructions sheds light on the mechanisms these machines use to carry out their functions; for example, in motility, interactions with other organisms and pathogenicity.
-
Continuing technological developments are increasing the quality of ECT data. Combined with other structure determination methods and fluorescence microscopy, ECT is moving us towards the ultimate goal: a complete understanding of the structure and location of every protein in a prokaryotic cell.
Abstract
Electron cryotomography (ECT) enables intact cells to be visualized in 3D in an essentially native state to 'macromolecular' (∼4 nm) resolution, revealing the basic architectures of complete nanomachines and their arrangements in situ. Since its inception, ECT has advanced our understanding of many aspects of prokaryotic cell biology, from morphogenesis to subcellular compartmentalization and from metabolism to complex interspecies interactions. In this Review, we highlight how ECT has provided structural and mechanistic insights into the physiology of bacteria and archaea and discuss prospects for the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
30 November 2016
It has recently come to the authors' attention that this Review is the first place in which Figure 1 was published. Yi-Wei Chang, who collected the data for Figure 1 and helped to revise the text, should have been included as an author. Therefore, on the title page, the corrected author list should read Catherine M. Oikonomou, Yi-Wei Chang and Grant J. Jensen
References
Loewy, A. G. & Siekevitz, P. Cell Structure and Function (Brooks/Cole, 1991).
Alberts, B. et al. Molecular Biology of the Cell (Garland Publishing, 1983).
Goodsell, D. S. Escherichia coli. Biochem. Mol. Biol. Educ. 37, 325–332 (2009).
Alberts, B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell 92, 291–294 (1998).
Pilhofer, M., Ladinsky, M. S., McDowall, A. W. & Jensen, G. J. Bacterial TEM: new insights from cryo-microscopy. Methods Cell Biol. 96, 21–45 (2010).
Pilhofer, M. et al. Architecture and host interface of environmental chlamydiae revealed by electron cryotomography. Environ. Microbiol. 16, 417–429 (2014).
Dubochet, J. et al. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21, 129–228 (1988).
Gan, L. & Jensen, G. J. Electron tomography of cells. Q. Rev. Biophys. 45, 27–56 (2012).
Lucic, V., Rigort, A. & Baumeister, W. Cryo-electron tomography: the challenge of doing structural biology in situ. J. Cell Biol. 202, 407–419 (2013).
Fridman, K., Mader, A., Zwerger, M., Elia, N. & Medalia, O. Advances in tomography: probing the molecular architecture of cells. Nat. Rev. Mol. Cell Biol. 13, 736–742 (2012).
Jensen, G. J. & Briegel, A. How electron cryotomography is opening a new window onto prokaryotic ultrastructure. Curr. Opin. Struct. Biol. 17, 260–267 (2007).
Zuber, B. et al. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 190, 5672–5680 (2008).
Hoffmann, C., Leis, A., Niederweis, M., Plitzko, J. M. & Engelhardt, H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl Acad. Sci. USA 105, 3963–3967 (2008).
Gan, L., Chen, S. & Jensen, G. J. Molecular organization of Gram-negative peptidoglycan. Proc. Natl Acad. Sci. USA 105, 18953–18957 (2008).
Beeby, M., Gumbart, J. C., Roux, B. & Jensen, G. J. Architecture and assembly of the Gram-positive cell wall. Mol. Microbiol. 88, 664–672 (2013).
Tocheva, E. I. et al. Peptidoglycan transformations during Bacillus subtilis sporulation. Mol. Microbiol. 88, 673–686 (2013).
Turner, R. D., Vollmer, W. & Foster, S. J. Different walls for rods and balls: the diversity of peptidoglycan. Mol. Microbiol. 91, 862–874 (2014).
Pilhofer, M. et al. Discovery of chlamydial peptidoglycan reveals bacteria with murein sacculi but without FtsZ. Nat. Commun. 4, 2856 (2013).
Swulius, M. T. et al. Long helical filaments are not seen encircling cells in electron cryotomograms of rod-shaped bacteria. Biochem. Biophys. Res. Commun. 407, 650–655 (2011).
Swulius, M. T. & Jensen, G. J. The helical MreB cytoskeleton in Escherichia coli MC1000/pLE7 is an artifact of the N-terminal yellow fluorescent protein tag. J. Bacteriol. 194, 6382–6386 (2012).
Dominguez-Escobar, J. et al. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science 333, 225–228 (2011).
Garner, E. C. et al. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science 333, 222–225 (2011).
Briegel, A. et al. Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography. Mol. Microbiol. 62, 5–14 (2006).
Ingerson-Mahar, M., Briegel, A., Werner, J. N., Jensen, G. J. & Gitai, Z. The metabolic enzyme CTP synthase forms cytoskeletal filaments. Nat. Cell Biol. 12, 739–746 (2010).
Barry, R. M. & Gitai, Z. Self-assembling enzymes and the origins of the cytoskeleton. Curr. Opin. Microbiol. 14, 704–711 (2011).
Kuhn, J. et al. Bactofilins, a ubiquitous class of cytoskeletal proteins mediating polar localization of a cell wall synthase in Caulobacter crescentus. EMBO J. 29, 327–339 (2010).
Scheffel, A. et al. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 440, 110–114 (2006).
Komeili, A., Li, Z., Newman, D. K. & Jensen, G. J. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311, 242–245 (2006). Together with Ref. 27, these papers describe the cytoskeletal filaments of MamK that align magnetosomes into chains.
Scheffel, A. & Schuler, D. The acidic repetitive domain of the Magnetospirillum gryphiswaldense MamJ protein displays hypervariability but is not required for magnetosome chain assembly. J. Bacteriol. 189, 6437–6446 (2007).
Katzmann, E., Scheffel, A., Gruska, M., Plitzko, J. M. & Schuler, D. Loss of the actin-like protein MamK has pleiotropic effects on magnetosome formation and chain assembly in Magnetospirillum gryphiswaldense. Mol. Microbiol. 77, 208–224 (2010).
Draper, O. et al. MamK, a bacterial actin, forms dynamic filaments in vivo that are regulated by the acidic proteins MamJ and LimJ. Mol. Microbiol. 82, 342–354 (2011).
Jenkins, C. et al. Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter. Proc. Natl Acad. Sci. USA 99, 17049–17054 (2002).
Sontag, C. A., Staley, J. T. & Erickson, H. P. In vitro assembly and GTP hydrolysis by bacterial tubulins BtubA and BtubB. J. Cell Biol. 169, 233–238 (2005).
Pilhofer, M., Ladinsky, M. S., McDowall, A. W., Petroni, G. & Jensen, G. J. Microtubules in bacteria: Ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton. PLoS Biol. 9, e1001213 (2011). This work describes bacterial microtubules containing five protofilaments.
Bohm, J. et al. Toward detecting and identifying macromolecules in a cellular context: template matching applied to electron tomograms. Proc. Natl Acad. Sci. USA 97, 14245–14250 (2000).
Frangakis, A. S. et al. Identification of macromolecular complexes in cryoelectron tomograms of phantom cells. Proc. Natl Acad. Sci. USA 99, 14153–14158 (2002).
Seybert, A., Herrmann, R. & Frangakis, A. S. Structural analysis of Mycoplasma pneumoniae by cryo-electron tomography. J. Struct. Biol. 156, 342–354 (2006).
Malmstrom, J. et al. Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans. Nature 460, 762–765 (2009).
Ortiz, J. O., Forster, F., Kurner, J., Linaroudis, A. A. & Baumeister, W. Mapping 70S ribosomes in intact cells by cryoelectron tomography and pattern recognition. J. Struct. Biol. 156, 334–341 (2006).
Comolli, L. R., Baker, B. J., Downing, K. H., Siegerist, C. E. & Banfield, J. F. Three-dimensional analysis of the structure and ecology of a novel, ultra-small archaeon. ISME J. 3, 159–167 (2009).
Beck, M. et al. Visual proteomics of the human pathogen Leptospira interrogans. Nat. Methods 6, 817–823 (2009).
Kuhner, S. et al. Proteome organization in a genome-reduced bacterium. Science 326, 1235–1240 (2009). Together with Ref. 41, this study uses visual proteomics to survey the in situ locations of protein complexes in intact cells.
Martinez-Planells, A. et al. Determination of the topography and biometry of chlorosomes by atomic force microscopy. Photosynth Res. 71, 83–90 (2002).
Psencik, J. et al. Structure of chlorosomes from the green filamentous bacterium Chloroflexus aurantiacus. J. Bacteriol. 191, 6701–6708 (2009).
Kudryashev, M., Aktoudianaki, A., Dedoglou, D., Stahlberg, H. & Tsiotis, G. The ultrastructure of Chlorobaculum tepidum revealed by cryo-electron tomography. Biochim. Biophys. Acta 1837, 1635–1642 (2014).
Ting, C. S., Hsieh, C., Sundararaman, S., Mannella, C. & Marko, M. Cryo-electron tomography reveals the comparative three-dimensional architecture of Prochlorococcus, a globally important marine cyanobacterium. J. Bacteriol. 189, 4485–4493 (2007).
Konorty, M., Kahana, N., Linaroudis, A., Minsky, A. & Medalia, O. Structural analysis of photosynthetic membranes by cryo-electron tomography of intact Rhodopseudomonas viridis cells. J. Struct. Biol. 161, 393–400 (2008).
Konorty, M. et al. Photosynthetic system in Blastochloris viridis revisited. Biochemistry 48, 4753–4761 (2009).
Tucker, J. D. et al. Membrane invagination in Rhodobacter sphaeroides is initiated at curved regions of the cytoplasmic membrane, then forms both budded and fully detached spherical vesicles. Mol. Microbiol. 76, 833–847 (2010).
Ellis, R. J. The most abundant protein in the world. Trends Biochem. Sci. 4, 241–244 (1979).
Schmid, M. F. et al. Structure of Halothiobacillus neapolitanus carboxysomes by cryo-electron tomography. J. Mol. Biol. 364, 526–535 (2006).
Iancu, C. V. et al. The structure of isolated Synechococcus strain WH8102 carboxysomes as revealed by electron cryotomography. J. Mol. Biol. 372, 764–773 (2007).
Iancu, C. V. et al. Organization, structure, and assembly of α-carboxysomes determined by electron cryotomography of intact cells. J. Mol. Biol. 396, 105–117 (2010).
Tocheva, E. I. et al. Structure and expression of propanediol utilization microcompartments in Acetonema longum. J. Bacteriol. 196, 1651–1658 (2014).
Butan, C. et al. Spiral architecture of the nucleoid in Bdellovibrio bacteriovorus. J. Bacteriol. 193, 1341–1350 (2011). This paper describes the organization of the nucleoid in an intact cell.
Raddi, G. et al. Three-dimensional structures of pathogenic and saprophytic Leptospira species revealed by cryo-electron tomography. J. Bacteriol. 194, 1299–1306 (2012).
Comolli, L. R. et al. A portable cryo-plunger for on-site intact cryogenic microscopy sample preparation in natural environments. Microsc. Res. Tech. 75, 829–836 (2012).
Luef, B. et al. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat. Commun. 6, 6372 (2015).
Schlimpert, S. et al. General protein diffusion barriers create compartments within bacterial cells. Cell 151, 1270–1282 (2012).
Comolli, L. R., Luef, B. & Chan, C. S. High-resolution 2D and 3D cryo-TEM reveals structural adaptations of two stalk-forming bacteria to an Fe-oxidizing lifestyle. Environ. Microbiol. 13, 2915–2929 (2011).
Luef, B. et al. Iron-reducing bacteria accumulate ferric oxyhydroxide nanoparticle aggregates that may support planktonic growth. ISME J. 7, 338–350 (2013).
Shetty, A., Chen, S., Tocheva, E. I., Jensen, G. J. & Hickey, W. J. Nanopods: a new bacterial structure and mechanism for deployment of outer membrane vesicles. PLoS ONE 6, e20725 (2011).
Murphy, G. E., Leadbetter, J. R. & Jensen, G. J. In situ structure of the complete Treponema primitia flagellar motor. Nature 442, 1062–1064 (2006).
Liu, J. et al. Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography: evidence for stator ring curvature and rotor/C-ring assembly flexion. J. Bacteriol. 191, 5026–5036 (2009).
Liu, J. et al. Cellular architecture of Treponema pallidum: novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography. J. Mol. Biol. 403, 546–561 (2010).
Chen, S. et al. Structural diversity of bacterial flagellar motors. EMBO J. 30, 2972–2981 (2011). This paper describes the structural diversity of flagellar motors in 11 bacterial species.
Hosogi, N., Shigematsu, H., Terashima, H., Homma, M. & Nagayama, K. Zernike phase contrast cryo-electron tomography of sodium-driven flagellar hook-basal bodies from Vibrio alginolyticus. J. Struct. Biol. 173, 67–76 (2011).
Zhao, X. et al. Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi. Proc. Natl Acad. Sci. USA 110, 14390–14395 (2013). This work uses high-resolution subtomogram averaging and mutant analysis to construct a detailed structural picture of flagellar assembly in vivo in B. burgdorferi.
Izard, J., Hsieh, C. E., Limberger, R. J., Mannella, C. A. & Marko, M. Native cellular architecture of Treponema denticola revealed by cryo-electron tomography. J. Struct. Biol. 163, 10–17 (2008).
Kudryashev, M. et al. Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes. Mol. Microbiol. 71, 1415–1434 (2009).
Xu, H., Raddi, G., Liu, J., Charon, N. W. & Li, C. Chemoreceptors and flagellar motors are subterminally located in close proximity at the two cell poles in spirochetes. J. Bacteriol. 193, 2652–2656 (2011).
Motaleb, M. A. et al. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl Acad. Sci. USA 97, 10899–10904 (2000).
Charon, N. W. et al. The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J. Bacteriol. 191, 600–607 (2009).
Sze, C. W. et al. Carbon storage regulator A (CsrA(Bb)) is a repressor of Borrelia burgdorferi flagellin protein FlaB. Mol. Microbiol. 82, 851–864 (2011).
Zhang, K., Tong, B. A., Liu, J. & Li, C. A single-domain FlgJ contributes to flagellar hook and filament formation in the Lyme disease spirochete Borrelia burgdorferi. J. Bacteriol. 194, 866–874 (2012).
Motaleb, M. A., Pitzer, J. E., Sultan, S. Z. & Liu, J. A novel gene inactivation system reveals altered periplasmic flagellar orientation in a Borrelia burgdorferi fliL mutant. J. Bacteriol. 193, 3324–3331 (2011).
Sultan, S. Z. et al. Motor rotation is essential for the formation of the periplasmic flagellar ribbon, cellular morphology, and Borrelia burgdorferi persistence within Ixodes scapularis tick and murine hosts. Infect. Immun. 83, 1765–1777 (2015).
Murphy, G. E., Matson, E. G., Leadbetter, J. R., Berg, H. C. & Jensen, G. J. Novel ultrastructures of Treponema primitia and their implications for motility. Mol. Microbiol. 67, 1184–1195 (2008).
Ruan, J. et al. Architecture of a flagellar apparatus in the fast-swimming magnetotactic bacterium MO-1. Proc. Natl Acad. Sci. USA 109, 20643–20648 (2012).
Liu, J., McBride, M. J. & Subramaniam, S. Cell surface filaments of the gliding bacterium Flavobacterium johnsoniae revealed by cryo-electron tomography. J. Bacteriol. 189, 7503–7506 (2007).
Kurner, J., Frangakis, A. S. & Baumeister, W. Cryo-electron tomography reveals the cytoskeletal structure of Spiroplasma melliferum. Science 307, 436–438 (2005). This early description of bacterial cytoskeletal filaments proposed a model for motility of helical Mollicutes based on cytoskeletal ribbons.
Henderson, G. P. & Jensen, G. J. Three-dimensional structure of Mycoplasma pneumoniae's attachment organelle and a model for its role in gliding motility. Mol. Microbiol. 60, 376–385 (2006).
Jasnin, M. et al. Three-dimensional architecture of actin filaments in Listeria monocytogenes comet tails. Proc. Natl Acad. Sci. USA 110, 20521–20526 (2013).
Byrne, M. E. et al. Desulfovibrio magneticus RS-1 contains an iron- and phosphorus-rich organelle distinct from its bullet-shaped magnetosomes. Proc. Natl Acad. Sci. USA 107, 12263–12268 (2010).
Abreu, F. et al. Cryo-electron tomography of the magnetotactic vibrio Magnetovibrio blakemorei: insights into the biomineralization of prismatic magnetosomes. J. Struct. Biol. 181, 162–168 (2013).
Zhang, P., Khursigara, C. M., Hartnell, L. M. & Subramaniam, S. Direct visualization of Escherichia coli chemotaxis receptor arrays using cryo-electron microscopy. Proc. Natl Acad. Sci. USA 104, 3777–3781 (2007).
Khursigara, C. M., Wu, X., Zhang, P., Lefman, J. & Subramaniam, S. Role of HAMP domains in chemotaxis signaling by bacterial chemoreceptors. Proc. Natl Acad. Sci. USA 105, 16555–16560 (2008).
Briegel, A. et al. Location and architecture of the Caulobacter crescentus chemoreceptor array. Mol. Microbiol. 69, 30–41 (2008).
Khursigara, C. M., Wu, X. & Subramaniam, S. Chemoreceptors in Caulobacter crescentus: trimers of receptor dimers in a partially ordered hexagonally packed array. J. Bacteriol. 190, 6805–6810 (2008).
Briegel, A., Beeby, M., Thanbichler, M. & Jensen, G. J. Activated chemoreceptor arrays remain intact and hexagonally packed. Mol. Microbiol. 82, 748–757 (2011).
Khursigara, C. M. et al. Lateral density of receptor arrays in the membrane plane influences sensitivity of the E. coli chemotaxis response. EMBO J. 30, 1719–1729 (2011).
Zhang, K. et al. Two CheW coupling proteins are essential in a chemosensory pathway of Borrelia burgdorferi. Mol. Microbiol. 85, 782–794 (2012).
Yamaichi, Y. et al. A multidomain hub anchors the chromosome segregation and chemotactic machinery to the bacterial pole. Genes Dev. 26, 2348–2360 (2012).
Briegel, A. et al. Universal architecture of bacterial chemoreceptor arrays. Proc. Natl Acad. Sci. USA 106, 17181–17186 (2009).
Briegel, A. et al. Structural conservation of chemotaxis machinery across Archaea and Bacteria. Environ. Microbiol. Rep. 7, 414–419 (2015).
Briegel, A. et al. Structure of bacterial cytoplasmic chemoreceptor arrays and implications for chemotactic signaling. eLife 3, e02151 (2014).
Briegel, A. et al. Bacterial chemoreceptor arrays are hexagonally packed trimers of receptor dimers networked by rings of kinase and coupling proteins. Proc. Natl Acad. Sci. USA 109, 3766–3771 (2012).
Liu, J. et al. Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells. Proc. Natl Acad. Sci. USA 109, E1481–E1488 (2012). Together with Ref. 97, this study provides a detailed structural model of the bacterial chemoreceptor array.
Briegel, A. et al. The mobility of two kinase domains in the Escherichia coli chemoreceptor array varies with signalling state. Mol. Microbiol. 89, 831–841 (2013).
Briegel, A. et al. New insights into bacterial chemoreceptor array structure and assembly from electron cryotomography. Biochemistry 53, 1575–1585 (2014).
Ebersbach, G., Briegel, A. & Jensen, G. J. & Jacobs-Wagner, C. A self-associating protein critical for chromosome attachment, division, and polar organization in Caulobacter. Cell 134, 956–968 (2008).
Bowman, G. R. et al. Caulobacter PopZ forms a polar subdomain dictating sequential changes in pole composition and function. Mol. Microbiol. 76, 173–189 (2010).
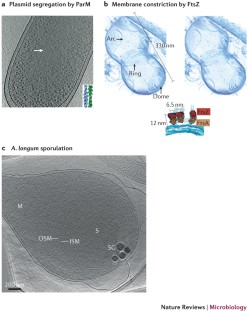
Salje, J., Zuber, B. & Lowe, J. Electron cryomicroscopy of E. coli reveals filament bundles involved in plasmid DNA segregation. Science 323, 509–512 (2009).
Bharat, T. A., Murshudov, G. N., Sachse, C. & Lowe, J. Structures of actin-like ParM filaments show architecture of plasmid-segregating spindles. Nature 523, 106–110 (2015). This study reveals plasmid segregation by antiparallel doublets of ParM filaments.
Aylett, C. H., Wang, Q., Michie, K. A., Amos, L. A. & Lowe, J. Filament structure of bacterial tubulin homologue TubZ. Proc. Natl Acad. Sci. USA 107, 19766–19771 (2010).
Szwedziak, P., Wang, Q., Freund, S. M. & Lowe, J. FtsA forms actin-like protofilaments. EMBO J. 31, 2249–2260 (2012).
Judd, E. M. et al. Distinct constrictive processes, separated in time and space, divide Caulobacter inner and outer membranes. J. Bacteriol. 187, 6874–6882 (2005).
Li, Z., Trimble, M. J., Brun, Y. V. & Jensen, G. J. The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J. 26, 4694–4708 (2007).
Lu, C., Reedy, M. & Erickson, H. P. Straight and curved conformations of FtsZ are regulated by GTP hydrolysis. J. Bacteriol. 182, 164–170 (2000).
Szwedziak, P., Wang, Q., Bharat, T. A., Tsim, M. & Lowe, J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. eLife 3, e04601 (2014).
Ghosal, D., Trambaiolo, D., Amos, L. A. & Lowe, J. MinCD cell division proteins form alternating copolymeric cytomotive filaments. Nat. Commun. 5, 5341 (2014).
Moll, A., Schlimpert, S., Briegel, A. & Jensen, G. J. & Thanbichler, M. DipM, a new factor required for peptidoglycan remodelling during cell division in Caulobacter crescentus. Mol. Microbiol. 77, 90–107 (2010).
Goley, E. D., Comolli, L. R., Fero, K. E., Downing, K. H. & Shapiro, L. DipM links peptidoglycan remodelling to outer membrane organization in Caulobacter. Mol. Microbiol. 77, 56–73 (2010).
Dobro, M. J. et al. Electron cryotomography of ESCRT assemblies and dividing Sulfolobus cells suggests that spiraling filaments are involved in membrane scission. Mol. Biol. Cell 24, 2319–2327 (2013).
Katzmann, E. et al. Magnetosome chains are recruited to cellular division sites and split by asymmetric septation. Mol. Microbiol. 82, 1316–1329 (2011).
Comolli, L. R., Kundmann, M. & Downing, K. H. Characterization of intact subcellular bodies in whole bacteria by cryo-electron tomography and spectroscopic imaging. J. Microsc. 223, 40–52 (2006).
Tocheva, E. I. et al. Polyphosphate storage during sporulation in the Gram-negative bacterium Acetonema longum. J. Bacteriol. 195, 3940–3946 (2013).
Beeby, M., Cho, M., Stubbe, J. & Jensen, G. J. Growth and localization of polyhydroxybutyrate granules in Ralstonia eutropha. J. Bacteriol. 194, 1092–1099 (2012).
Toso, D. B., Henstra, A. M., Gunsalus, R. P. & Zhou, Z. H. Structural mass and elemental analyses of storage granules in methanogenic archaeal cells. Environ. Microbiol. 13, 2587–2599 (2011).
Tocheva, E. I. et al. Peptidoglycan remodeling and conversion of an inner membrane into an outer membrane during sporulation. Cell 146, 799–812 (2011). This work reveals the interconversion of inner and outer membranes during Gram-negative sporulation, suggesting a potential evolutionary source of the outer membrane.
Schrempf, H., Koebsch, I., Walter, S., Engelhardt, H. & Meschke, H. Extracellular Streptomyces vesicles: amphorae for survival and defence. Microb. Biotechnol. 4, 286–299 (2011).
Sleytr, U. B., Messner, P., Pum, D. & Sara, M. Crystalline bacterial cell surface layers. Mol. Microbiol. 10, 911–916 (1993).
Grimm, R. et al. Electron tomography of ice-embedded prokaryotic cells. Biophys. J. 74, 1031–1042 (1998).
Amat, F. et al. Analysis of the intact surface layer of Caulobacter crescentus by cryo-electron tomography. J. Bacteriol. 192, 5855–5865 (2010).
Nickell, S. Hegerl, R., Baumeister, W. & Rachel, R. Pyrodictium cannulae enter the periplasmic space but do not enter the cytoplasm, as revealed by cryo-electron tomography. J. Struct. Biol. 141, 34–42 (2003).
Junglas, B. et al. Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell-cell interaction, and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography. Arch. Microbiol. 190, 395–408 (2008).
Comolli, L. R. & Banfield, J. F. Inter-species interconnections in acid mine drainage microbial communities. Front. Microbiol. 5, 367 (2014).
Moissl, C. Rachel, R., Briegel, A., Engelhardt, H. & Huber, R. The unique structure of archaeal 'hami', highly complex cell appendages with nano-grappling hooks. Mol. Microbiol. 56, 361–370 (2005).
Basler, M., Pilhofer, M., Henderson, G. P., Jensen, G. J. & Mekalanos, J. J. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483, 182–186 (2012). This study reveals the phage tail-like contractile mechanism of the T6SS.
Chang, Y. W. et al. Correlated cryogenic photoactivated localization microscopy and cryo-electron tomography. Nat. Methods 11, 737–739 (2014). This paper describes the technique of correlated cryo-PALM and ECT.
Shikuma, N. J. et al. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 343, 529–533 (2014).
Borgnia, M. J., Subramaniam, S. & Milne, J. L. Three-dimensional imaging of the highly bent architecture of Bdellovibrio bacteriovorus by using cryo-electron tomography. J. Bacteriol. 190, 2588–2596 (2008).
Muller, A. et al. Ultrastructure and complex polar architecture of the human pathogen Campylobacter jejuni. Microbiologyopen 3, 702–710 (2014).
Izard, J. et al. Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete. J. Bacteriol. 191, 7566–7580 (2009).
Kudryashev, M. et al. Evidence of direct cell-cell fusion in Borrelia by cryogenic electron tomography. Cell. Microbiol. 13, 731–741 (2011).
Abrusci, P. et al. Architecture of the major component of the type III secretion system export apparatus. Nat. Struct. Mol. Biol. 20, 99–104 (2013).
Kawamoto, A. et al. Common and distinct structural features of Salmonella injectisome and flagellar basal body. Sci. Rep. 3, 3369 (2013).
Kudryashev, M. et al. In situ structural analysis of the Yersinia enterocolitica injectisome. eLife 2, e00792 (2013).
Kudryashev, M. et al. Yersinia enterocolitica type III secretion injectisomes form regularly spaced clusters, which incorporate new machines upon activation. Mol. Microbiol. 95, 875–884 (2014).
Nans, A., Saibil, H. R. & Hayward, R. D. Pathogen–host reorganization during Chlamydia invasion revealed by cryo-electron tomography. Cell. Microbiol. 16, 1457–1472 (2014).
Hu, B. et al. Visualization of the type III secretion sorting platform of Shigella flexneri. Proc. Natl Acad. Sci. USA 112, 1047–1052 (2015). This work provides a pseudo-atomic model of the pathogenic T3SS, including the substrate sorting platform.
Huang, Z. et al. Cryo-electron tomography of Chlamydia trachomatis gives a clue to the mechanism of outer membrane changes. J. Electron. Microsc. (Tokyo) 59, 237–241 (2010).
Wilkat, M., Herdoiza, E., Forsbach-Birk, V., Walther, P. & Essig, A. Electron tomography and cryo-SEM characterization reveals novel ultrastructural features of host-parasite interaction during Chlamydia abortus infection. Histochem. Cell Biol. 142, 171–184 (2014).
LaRocca, T. J. et al. The bactericidal effect of a complement-independent antibody is osmolytic and specific to Borrelia. Proc. Natl Acad. Sci. USA 106, 10752–10757 (2009).
Watkins, B. Y. et al. Mycobacterium marinum SecA2 promotes stable granulomas and induces tumor necrosis factor-α in vivo. Infect. Immun. 80, 3512–3520 (2012).
Koning, R. I. et al. Cryo-electron tomography analysis of membrane vesicles from Acinetobacter baumannii ATCC19606 T. Res. Microbiol. 164, 397–405 (2013).
Trepout, S. et al. Structure of reconstituted bacterial membrane efflux pump by cryo-electron tomography. Biochim. Biophys. Acta 1798, 1953–1960 (2010).
Wysocki, L. M. & Lavis, L. D. Advances in the chemistry of small molecule fluorescent probes. Curr. Opin. Chem. Biol. 15, 752–759 (2011).
Zhang, W. H., Otting, G. & Jackson, C. J. Protein engineering with unnatural amino acids. Curr. Opin. Struct. Biol. 23, 581–587 (2013).
Mercogliano, C. P. & DeRosier, D. J. Concatenated metallothionein as a clonable gold label for electron microscopy. J. Struct. Biol. 160, 70–82 (2007).
Wang, Q., Mercogliano, C. P. & Lowe, J. A ferritin-based label for cellular electron cryotomography. Structure 19, 147–154 (2011).
Diestra, E., Fontana, J., Guichard, P., Marco, S. & Risco, C. Visualization of proteins in intact cells with a clonable tag for electron microscopy. J. Struct. Biol. 165, 157–168 (2009).
Bouchet-Marquis, C., Pagratis, M., Kirmse, R. & Hoenger, A. Metallothionein as a clonable high-density marker for cryo-electron microscopy. J. Struct. Biol. 177, 119–127 (2012).
Delgado, L. Martinez, G., Lopez-Iglesias, C. & Mercade, E. Cryo-electron tomography of plunge-frozen whole bacteria and vitreous sections to analyze the recently described bacterial cytoplasmic structure, the Stack. J. Struct. Biol. 189, 220–229 (2015).
Bernadac, A. et al. Structural properties of the tubular appendage spinae from marine bacterium Roseobacter sp. strain YSCB. Sci. Rep. 2, 950 (2012).
Tivol, W. F., Briegel, A. & Jensen, G. J. An improved cryogen for plunge freezing. Microsc. Microanal. 14, 375–379 (2008).
Al-Amoudi, A., Norlen, L. P. & Dubochet, J. Cryo-electron microscopy of vitreous sections of native biological cells and tissues. J. Struct. Biol. 148, 131–135 (2004). This paper describes the technique of vitreous cryosectioning to expand the power of ECT to thicker biological samples.
Al-Amoudi, A., Studer, D. & Dubochet, J. Cutting artefacts and cutting process in vitreous sections for cryo-electron microscopy. J. Struct. Biol. 150, 109–121 (2005).
Dubochet, J. et al. How to “read” a vitreous section. Methods Cell Biol. 79, 385–406 (2007).
Marko, M., Hsieh, C., Schalek, R., Frank, J. & Mannella, C. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat. Methods 4, 215–217 (2007).
Rigort, A. et al. Focused ion beam micromachining of eukaryotic cells for cryoelectron tomography. Proc. Natl Acad. Sci. USA 109, 4449–4454 (2012). This paper illustrates the power of FIB milling to enable high-resolution ECT of thick cells without the artefacts introduced by vitreous cryosectioning.
Rigort, A., Villa, E., Bauerlein, F. J., Engel, B. D. & Plitzko, J. M. Integrative approaches for cellular cryo-electron tomography: correlative imaging and focused ion beam micromachining. Methods Cell Biol. 111, 259–281 (2012).
Strunk, K. M., Wang, K., Ke, D., Gray, J. L. & Zhang, P. Thinning of large mammalian cells for cryo-TEM characterization by cryo-FIB milling. J. Microsc. 247, 220–227 (2012).
McMullan, G., Clark, A. T., Turchetta, R. & Faruqi, A. R. Enhanced imaging in low dose electron microscopy using electron counting. Ultramicroscopy 109, 1411–1416 (2009).
Murata, K. et al. Zernike phase contrast cryo-electron microscopy and tomography for structure determination at nanometer and subnanometer resolutions. Structure 18, 903–912 (2010).
Dai, W. et al. Zernike phase-contrast electron cryotomography applied to marine cyanobacteria infected with cyanophages. Nat. Protoc. 9, 2630–2642 (2014). This study uses Zernike phase contrast ECT to describe the cyanophage maturation process inside cyanobacterial cells.
Fukuda, Y., Laugks, U., Lucic, V., Baumeister, W. & Danev, R. Electron cryotomography of vitrified cells with a Volta phase plate. J. Struct. Biol. 190, 143–154 (2015).
Danev, R., Buijsse, B., Khoshouei, M., Plitzko, J. M. & Baumeister, W. Volta potential phase plate for in-focus phase contrast transmission electron microscopy. Proc. Natl Acad. Sci. USA 111, 15635–15640 (2014). This paper describes the Volta phase plate for improving contrast in transmission electron microscopy imaging.
Asano, S. et al. Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science 347, 439–442 (2015). This paper illustrates the power of Volta phase plate ECT, distinguishing conformational states of a protein complex in an intact eukaryotic cell.
Hu, G. B., Wei, H., Rice, W. J., Stokes, D. L. & Gottlieb, P. Electron cryo-tomographic structure of cystovirus Φ12. Virology 372, 1–9 (2008).
Leo-Macias, A. et al. Toroidal surface complexes of bacteriophage Φ12 are responsible for host-cell attachment. Virology 414, 103–109 (2011).
Chang, J. T. et al. Visualizing the structural changes of bacteriophage ɛ15 and its Salmonella host during infection. J. Mol. Biol. 402, 731–740 (2010).
Dai, W. et al. Three-dimensional structure of tropism-switching Bordetella bacteriophage. Proc. Natl Acad. Sci. USA 107, 4347–4352 (2010).
Hu, B., Margolin, W., Molineux, I. J. & Liu, J. The bacteriophage T7 virion undergoes extensive structural remodeling during infection. Science 339, 576–579 (2013).
Parent, K. N. et al. OmpA and OmpC are critical host factors for bacteriophage Sf6 entry in Shigella. Mol. Microbiol. 92, 47–60 (2014).
Guerrero-Ferreira, R. C. et al. Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc. Natl Acad. Sci. USA 108, 9963–9968 (2011).
Dent, K. C. et al. The asymmetric structure of an icosahedral virus bound to its receptor suggests a mechanism for genome release. Structure 21, 1225–1234 (2013).
Bohm, J. et al. FhuA-mediated phage genome transfer into liposomes: a cryo-electron tomography study. Curr. Biol. 11, 1168–1175 (2001). This early application of ECT reveals the contractile injection mechanism of the phage tail.
Liu, X. et al. Structural changes in a marine podovirus associated with release of its genome into Prochlorococcus. Nat. Struct. Mol. Biol. 17, 830–836 (2010).
Fu, X., Walter, M. H., Paredes, A., Morais, M. C. & Liu, J. The mechanism of DNA ejection in the Bacillus anthracis spore-binding phage 8a revealed by cryo-electron tomography. Virology 421, 141–148 (2011).
Liu, J., Chen, C. Y., Shiomi, D., Niki, H. & Margolin, W. Visualization of bacteriophage P1 infection by cryo-electron tomography of tiny Escherichia coli. Virology 417, 304–311 (2011).
Nemecek, D., Heymann, J. B., Qiao, J., Mindich, L. & Steven, A. C. Cryo-electron tomography of bacteriophage Φ6 procapsids shows random occupancy of the binding sites for RNA polymerase and packaging NTPase. J. Struct. Biol. 171, 389–396 (2010).
Dai, W. et al. Visualizing virus assembly intermediates inside marine cyanobacteria. Nature 502, 707–710 (2013).
Nemecek, D. et al. Stepwise expansion of the bacteriophage Φ6 procapsid: possible packaging intermediates. J. Mol. Biol. 414, 260–271 (2011).
Dewey, J. S. et al. Micron-scale holes terminate the phage infection cycle. Proc. Natl Acad. Sci. USA 107, 2219–2223 (2010).
Quemin, E. R. et al. First insights into the entry process of hyperthermophilic archaeal viruses. J. Virol. 87, 13379–13385 (2013).
Fu, C. Y. et al. In vivo assembly of an archaeal virus studied with whole-cell electron cryotomography. Structure 18, 1579–1586 (2010).
Daum, B. et al. Self-assembly of the general membrane-remodeling protein PVAP into sevenfold virus-associated pyramids. Proc. Natl Acad. Sci. USA 111, 3829–3834 (2014). This study reveals the lytic mechanism of pyramidal archaeal viruses.
Hong, C. et al. Lemon-shaped halo archaeal virus His1 with uniform tail but variable capsid structure. Proc. Natl Acad. Sci. USA 112, 2449–2454 (2015).
Acknowledgements
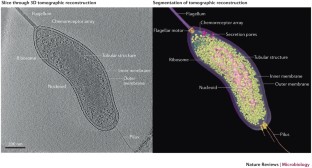
The authors apologize that they could not discuss all of the work in this burgeoning field. The authors thank members of the Jensen laboratory for helpful comments on the manuscript, and J. Ding and Y.-W. Chang for producing the accompanying movie. The authors also thank L. Sockett (University of Nottingham) for the gift of the Bdellovibrio bacteriovorus strain imaged in figure 1 and shown in the accompanying movie. Microbial electron cryotomography (ECT) in the Jensen laboatory is supported, in part, by the Howard Hughes Medical Institute, the US National Institutes of Health (grants RO1 GM101425 and RO1 GM094800), the Beckman Institute at Caltech, Caltech's Center for Environmental Microbial Interactions, and gifts to Caltech from the Gordon and Betty Moore Foundation and the Agouron Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (movie)
An intact Bdellovibrio bacteriovorus cell in standard media was plunge-frozen and imaged by ECT. The resulting tilt-series of images was reconstructed into a 3D tomogram. The movie shows the full reconstruction and segmentation, as well as fitting of crystal structures into EM densities. (MOV 190651 kb)
Glossary
- Phase plates
-
Electron microscope components that shift the phases of the scattered beam with respect to the unscattered beam to boost contrast.
- Vitreous cryosectioning
-
A sample preparation technique in which thick samples are frozen rapidly to prevent ice formation and then cut with a diamond blade into thin (50–400 nm) slices that can be imaged by electron cryotomography.
- Peptidoglycan
-
A polymer crosslinked into a mesh-like network that forms the cell walls of most bacteria.
- Sacculi
-
Sac-like exoskeletons (peptidoglycan cell walls) of bacterial cells.
- Stalked cell
-
In the context of Caulobacter crescentus, one of the two cells produced by asymmetric division. Unlike the motile swarmer cell, the stalked cell is attached to a surface and is capable of replication and division. Swarmer cells later differentiate into stalked cells.
- Magnetosomes
-
Compartments containing ferrous microcrystals that are used by magnetotactic bacteria to orient the cell in a magnetic field.
- Template matching
-
A digital image processing technique to search a 3D tomogram for an object of interest (the template). The template is typically a single-particle reconstruction or X-ray crystallographical structure of a macromolecule or complex of interest.
- Archaeal Richmond Mine acidophilic nanoorganisms
-
(ARMAN). A highly divergent group of archaea isolated from an extremely acidic environment in California.
- Reaction centres
-
Complexes of enzymes, pigments and cofactors that convert solar energy that is captured by light-harvesting antennae into chemical energy during photosynthesis.
- Correlated light microscopy and ECT
-
A specialization of correlated light microscopy and electron microscopy (CLEM) that is used to locate structures of interest within visually crowded tomograms. Cells containing a fluorescently labelled target protein are rapidly frozen on electron microscope grids that contain landmarks for correlation, and first imaged by light microscopy to identify the location of fluorescent signals. Grids are then transferred to the electron microscope and the same locations are imaged at high resolution by electron cryotomography (ECT).
- Electron microscopy docking
-
A method for modelling the structure of macromolecular complexes by fitting high-resolution models (like X-ray crystallographical structures) of components into electron microscopy density maps, often informed by biochemical studies detailing component interactions.
- Subtomogram averaging
-
An image processing technique to increase the clarity of structures that are present in multiple copies and/or in multiple tomograms. Averaging particles increases the signal-to-noise ratio and can yield reliable high-resolution (<1 nm) detail, including secondary protein structure.
- Endosomal-sorting complexes required for transport
-
(ESCRT). Protein complexes that remodel cellular membranes to carry out various processes, including cell division and viral budding.
- Diderm cell plan
-
Under a classification system not based on the Gram stain, diderm bacteria are surrounded by two lipid bilayer membranes, as opposed to monoderms, which have only one.
- Correlated cryo-PALM and ECT
-
A specialized application of correlated light microscopy and electron cryotomography (ECT) using a super-resolution microscopy technique — photoactivated localization microscopy (PALM) — to increase the localization precision of a structure of interest in fluorescence images for correlation with high-resolution ECT images.
- Pyocins
-
Type VI secretion system (T6SS)-like contractile nanomachines, related to phage tails, that bacteria use to kill other bacteria.
- Elementary bodies
-
The non-replicating forms of Chlamydia spp., which are responsible for cellular infection.
- Reticulate bodies
-
The metabolically active forms of Chlamydia spp., which are found in the cytoplasm of infected cells.
- Single-particle reconstruction
-
A transmission electron microscopy technique in which many identical copies of a purified macromolecule or complex are imaged, providing different projection views of the particle that can then be computationally combined into a 3D reconstruction.
Rights and permissions
About this article
Cite this article
Oikonomou, C., Chang, YW. & Jensen, G. A new view into prokaryotic cell biology from electron cryotomography. Nat Rev Microbiol 14, 205–220 (2016). https://doi.org/10.1038/nrmicro.2016.7
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2016.7
This article is cited by
-
Accurate real space iterative reconstruction (RESIRE) algorithm for tomography
Scientific Reports (2023)
-
Bridging nano- and microscale X-ray tomography for battery research by leveraging artificial intelligence
Nature Nanotechnology (2022)
-
Raman microspectroscopy for microbiology
Nature Reviews Methods Primers (2021)
-
A unified framework for packing deformable and non-deformable subcellular structures in crowded cryo-electron tomogram simulation
BMC Bioinformatics (2020)
-
A simulated annealing approach for resolution guided homogeneous cryo‐electron microscopy image selection
Quantitative Biology (2020)



