Key Points
-
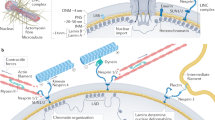
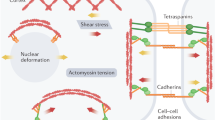
Cellular mechanical states modulate cytoskeleton–nucleus links and trigger the translocation of regulatory molecules to the nucleus.
-
The remodelling of cytoskeleton–nucleus links results in distinct morphological as well as mechanical and dynamic properties of the cell nucleus.
-
The cell type-specific organization of chromosomes and their intermingling is modulated by the mechanical state of a cell.
-
The recruitment of transcription factors to their target genes is facilitated by the nuclear mechanical state through the establishment of particular chromosome neighbourhoods and functional gene clusters.
-
Such cell type-specific chromosome neighbourhoods and gene clusters are established during cell differentiation.
-
The spatial organization of chromosomes and their intermingling are crucial for mechanoregulation of gene expression, and alterations thereof can result in the onset of various diseases.
Abstract
It is well established that cells sense chemical signals from their local microenvironment and transduce them to the nucleus to regulate gene expression programmes. Although a number of experiments have shown that mechanical cues can also modulate gene expression, the underlying mechanisms are far from clear. Nevertheless, we are now beginning to understand how mechanical cues are transduced to the nucleus and how they influence nuclear mechanics, genome organization and transcription. In particular, recent progress in super-resolution imaging, in genome-wide application of RNA sequencing, chromatin immunoprecipitation and chromosome conformation capture and in theoretical modelling of 3D genome organization enables the exploration of the relationship between cell mechanics, 3D chromatin configurations and transcription, thereby shedding new light on how mechanical forces regulate gene expression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Petridou, N. I., Spiró, Z. & Heisenberg, C. P. Multiscale force sensing in development. Nat. Cell Biol. 19, 581–588 (2017).
Kutys, M. L. & Chen, C. S. Forces and mechanotransduction in 3D vascular biology. Curr. Opin. Cell Biol. 42, 73–79 (2016).
Humphrey, J. D., Dufresne, E. R. & Schwartz, M. A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014).
Schreiber, T. H., Shinder, V., Cain, D. W., Alon, R. & Sackstein, R. Shear flow-dependent integration of apical and subendothelial chemokines in T-cell transmigration: implications for locomotion and the multistep paradigm. Blood 109, 1381–1386 (2007).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Przybyla, L., Muncie, J. M. & Weaver, V. M. Mechanical control of epithelial-to-mesenchymal transitions in development and cancer. Annu. Rev. Cell Dev. Biol. 32, 527–554 (2016).
Chin, L., Xia, Y., Discher, D. E. & Janmey, P. A. Mechanotransduction in cancer. Curr. Opin. Chem. Eng. 11, 77–84 (2016).
Brock, A., Krause, S. & Ingber, D. E. Control of cancer formation by intrinsic genetic noise and microenvironmental cues. Nat. Rev. Cancer 15, 499–509 (2015).
Sun, Z., Guo, S. S. & Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 215, 445–456 (2016).
Roca-Cusachs, P., Conte, V. & Trepat, X. Quantifying forces in cell biology. Nat. Cell Biol. 19, 742–751 (2017).
Stutchbury, B., Atherton, P., Tsang, R., Wang, D. Y. & Ballestrem, C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 130, 1612–1624 (2017).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Collins, C., Denisin, A. K., Pruitt, B. L. & Nelson, W. J. Changes in E-cadherin rigidity sensing regulate cell adhesion. Proc. Natl Acad. Sci. USA 114, E5835–E5844 (2017).
Luca, V. C. et al. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 355, 1320–1324 (2017).
Mattila, P. K., Batista, F. D. & Treanor, B. Dynamics of the actin cytoskeleton mediates receptor cross talk: an emerging concept in tuning receptor signaling. J. Cell Biol. 212, 267–280 (2016).
Moore, S. W., Roca-Cusachs, P. & Sheetz, M. P. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev. Cell 19, 194–206 (2010).
Engler, A. J. et al. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). This study demonstrates that mesenchymal stem cells can be differentiated into different lineages by changing the substrate rigidity.
Kilian, K. A., Bugarija, B., Lahn, B. T. & Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 4872–4877 (2010). This study identifies surface topography as a regulator of stem cell differentiation.
Adamo, L. et al. Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 (2009).
Fernández- Sánchez, M. E. et al. Mechanical induction of the tumorigenic β-catenin pathway by tumor growth pressure. Nature 523, 92–95 (2015).
Jain, R. K., Martin, J. D. & Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014).
Shivashankar, G. V. Mechanosignaling to the cell nucleus and gene regulation. Annu. Rev. Biophys. 40, 361–378 (2011).
Panciera, T., Azzolin, L., Cordenonsi, M. & Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. http://dx.doi.org/10.1038/nrm.2017.87 (2017).
Lanctôt, C. et al. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat. Rev. Genet. 8, 104–115 (2007).
Dekker, J. & Mirny, L. The 3D genome as moderator of chromosomal communication. Cell 164, 1110–1121 (2016).
Mammoto, A., Mammoto, T. & Ingber, D. E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 125, 3061–3073 (2012).
Wang, N., Tytell, J. D. & Ingber, D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82 (2009).
Kadrmas, J. L. & Beckerle, M. C. The LIM domain: from the cytoskeleton to the nucleus. Nat. Rev. Mol. Cell Biol. 5, 920–931 (2004).
Pawłowski, R. et al. An actin-regulated importin α/β-dependent extended bipartite NLS directs nuclear import of MRTF-A. EMBO J. 29, 3448–3458 (2010).
Starr, D. A. & Fridolfsson, H. N. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 26, 421–444 (2010).
Lombardi, M. L. et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 286, 26743–26753 (2011).
Crisp, M. et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41–53 (2006).
Alam, S. G. et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 6, 38063 (2016).
Arsenovic, P. T. et al. Nesprin-2G, a component of the nuclear LINC complex, is subject to Myosin-dependent tension. Biophys. J. 110, 34–43 (2016).
Lammerding, J. et al. Abnormal nuclear shape and impaired mechanotransduction in emerin deficient cells. J. Cell Biol. 170, 781–791 (2005).
Zhang, Q. et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery-Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16, 2816–2833 (2007).
Sosa, B. A., Kutay, U. & Schwartz, T. U. Structural insights into LINC complexes. Curr. Opin. Struct. Biol. 23, 285–291 (2013).
Iyer, K. V. et al. Mechanical activation of cells induces chromatin remodeling preceding MKL nuclear transport. Biophys. J. 103, 1416–1428 (2012).
Tajik, A. et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 15, 1287–1296 (2016). This study demonstrates the direct mechanical coupling between plasma membrane and chromatin for transcription control.
Li, Q., Makhija, E., Hameed, F. M. & Shivashankar, G. V. Micropillar displacements by cell traction forces are mechanically correlated with nuclear dynamics. Biochem. Biophys. Res. Commun. 461, 372–377 (2015).
Guilluy, C. et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 16, 376–381 (2014). This paper shows that the inner nuclear membrane protein emerin can be activated by mechanical forces.
Kumar, A. et al. ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 158, 633–646 (2014).
Enyedi, B., Jelcic, M. & Niethammer, P. The cell nucleus serves as a mechanotransducer of tissue damage-induced inflammation. Cell 165, 1160–1170 (2016).
Raab, M. et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 (2016).
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016).
Irianto, J. et al. DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol. 27, 210–223 (2017).
Kaunas, R., Nguyen, P., Usami, S. & Chien, S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl Acad. Sci. USA 102, 15895–15900 (2005).
Khatau, S. B. et al. A perinuclear actin cap regulates nuclear shape. Proc. Natl Acad. Sci. USA 106, 19017–19022 (2009). This paper identifies the role of apical actin stress fibres in regulating the shape of the cell nucleus, a process dependent on cell–extracellular matrix interactions.
Li, Q. et al. The regulation of dynamic mechanical coupling between actin cytoskeleton and nucleus by matrix geometry. Biomaterials 35, 961–969 (2014).
Shao, X., Li, Q., Mogilner, A., Bershadsky, A. D. & Shivashankar, G. V. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc. Natl Acad. Sci. USA 112, E2595–E2601 (2015).
Ramabhadran, V., Hatch, A. L. & Higgs, H. N. Actin monomers activate inverted formin 2 by competing with its autoinhibitory interaction. J. Biol. Chem. 288, 26847–26855 (2013).
Versaevel, M., Grevesse, T. & Gabriele, S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat. Commun. 3, 671 (2012).
Heo, S. J. et al. Mechanically induced chromatin condensation requires cellular contractility in mesenchymal stem cells. Biophys. J. 111, 864–874 (2016).
Gruenbaum, Y. & Foisner, R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 84, 131–164 (2015).
Thorpe, S. D. & Lee, D. A. Dynamic regulation of nuclear architecture and mechanics-a rheostatic role for the nucleus in tailoring cellular mechanosensitivity. Nucleus 8, 287–300 (2017).
Cho, S., Irianto, J. & Discher, D. E. Mechanosensing by the nucleus: from pathways to scaling relationships. J. Cell Biol. 216, 305–315 (2017).
Kim, D. H. & Wirtz, D. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials 48, 161–172 (2015).
Mazumder, A. & Shivashankar, G. V. Gold-nanoparticle-assisted laser perturbation of chromatin assembly reveals unusual aspects of nuclear architecture within living cells. Biophys. J. 93, 2209–2216 (2007).
Ramdas, N. M. & Shivashankar, G. V. Cytoskeletal control of nuclear morphology and chromatin organization. J. Mol. Biol. 427, 695–706 (2015).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013). This study shows, using a proteomic screen, that nuclear lamin A levels increase with increased tissue stiffness.
Burke, B. & Stewart, C. L. The nuclear lamins: flexibility in function. Nat. Rev. Mol. Cell Biol. 14, 13–24 (2013).
Buxboim, A. et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 24, 1909–1917 (2014).
Ihalainen, T. O. et al. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat. Mater. 14, 1252 (2015).
Bascom, G. & Schlick, T. Linking chromatin fibers to gene folding by hierarchical looping. Biophys. J. 112, 434–445 (2017).
Mazumder, A. et al. Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed nucleus. Biophys. J. 95, 3028–3035 (2008).
Allis, C. D. & Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500 (2016).
Gesson, K. et al. A-Type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res. 26, 462–473 (2016).
Schreiner, S. M., Koo, P. K., Zhao, Y., Mochrie, S. G. & King, M. C. The tethering of chromatin to the nuclear envelope supports nuclear mechanics. Nat. Commun. 6, 7159 (2015).
Stephens, A. D., Banigan, E. J., Adam, S. A., Goldman, R. D. & Marko, J. F. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 28, 1984–1996 (2017).
Hanson, L. et al. Vertical nanopillars for in situ probing of nuclear mechanics in adherent cells. Nat. Nanotechnol. 10, 554–562 (2015).
Radhakrishnan, A. V., Jokhun, D. S., Venkatachalapathy, S. & Shivashankar, G. V. Nuclear positioning and its translational dynamics are regulated by cell geometry. Biophys. J. 112, 1920–1928 (2017).
Kumar, A., Maitra, A., Sumit, M., Ramaswamy, S. & Shivashankar, G. V. Actomyosin contractility rotates the cell nucleus. Sci. Rep. 4, 3781 (2014).
Makhija, E., Jokhun, D. S. & Shivashankar, G. V. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc. Natl Acad. Sci. USA 113, E32–E40 (2016). This paper demonstrates that the cell–matrix interactions regulate nuclear and chromatin dynamics.
Ji, J. Y. et al. Cell nuclei spin in the absence of lamin b1. J. Biol. Chem. 282, 20015–20026 (2007).
Toh, K. C., Ramdas, N. M. & Shivashankar, G. V. Actin cytoskeleton differentially alters the dynamics of lamin A, HP1α and H2B core histone proteins to remodel chromatin condensation state in living cells. Integr. Biol. (Camb.) 7, 1309–1317 (2015).
Jain, N., Iyer, K. V., Kumar, A. & Shivashankar, G. V. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc. Natl Acad. Sci. USA 110, 11349–11354 (2013). This study exemplifies the coupling between cell shape and modular gene expression patterns by analysing transcriptome maps in polarized and isotropic cell shapes.
Pajerowski, J. D., Dahl, K. N., Zhong, F. L., Sammak, P. J. & Discher, D. E. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl Acad. Sci. USA 104, 15619–15624 (2007).
Meshorer, E. et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell 10, 105–116 (2006).
Bhattacharya, D., Talwar, S., Mazumder, A. & Shivashankar, G. V. Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys. J. 96, 3832–3839 (2009).
Talwar, S., Kumar, A., Rao, M., Menon, G. I. & Shivashankar, G. V. Correlated spatio-temporal fluctuations in chromatin compaction states characterize stem cells. Biophys. J. 104, 553–564 (2013).
Mazumder, A. & Shivashankar, G. V. Emergence of a prestressed eukaryotic nucleus during cellular differentiation and development. J. R. Soc. Interface 7, S321–S330 (2010).
Heo, S. J. et al. Differentiation alters stem cell nuclear architecture, mechanics, and mechano-sensitivity. eLife 5, e18207 (2016).
Wang, Y. et al. Visualizing the mechanical activation of Src. Nature 434, 1040–1045 (2005).
Vartiainen, M. K., Guettler, S., Larijani, B. & Treisman, R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 (2007). This study shows that cytoplasmic-to-nuclear shuttling of the transcription cofactor MAL (also known as MRTF) is regulated by the actin polymerization state.
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). This study demonstrates the importance of YAP–TAZ transcription factors in cellular nuclear mechanosensing and the downstream differential regulation of transcriptional programmes.
Totaro, A. et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat. Commun. 8, 15206 (2017).
Le, H. Q. et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 18, 864–875 (2016).
Speight, P., Kofler, M., Szászi, K. & Kapus, A. Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFβ-regulated Smad3. Nat. Commun. 7, 11642 (2016).
Qi, Y. X. et al. Nuclear envelope proteins modulate proliferation of vascular smooth muscle cells during cyclic stretch application. Proc. Natl Acad. Sci. USA 113, 5293–5298 (2016).
Nakazawa, N. et al. Matrix mechanics controls FHL2 movement to the nucleus to activate p21 expression. Proc. Natl Acad. Sci. USA 113, E6813–E6822 (2016).
Thomas, C. H., Collier, J. H., Sfeir, C. S. & Healy, K. E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl Acad. Sci. USA 99, 1972–1977 (2002).
Tang, R. H. et al. Myocardin inhibits cellular proliferation by inhibiting NF-κB(p65)-dependent cell cycle progression. Proc. Natl Acad. Sci. USA 105, 3362–3367 (2008).
Mitra, A. et al. Cell geometry dictates TNFα-induced genome response. Proc. Natl Acad. Sci. USA 114, E3882–E3891 (2017). This study exemplifies the role of the cellular and nuclear mechanical state for integrating biochemical signals to regulate gene expression.
Maharana, S. et al. Chromosome intermingling-the physical basis of chromosome organization in differentiated cells. Nucleic Acids Res. 44, 5148–5160 (2016).
Wang, Y., Nagarajan, M., Uhler, C. & Shivashankar, G. V. Orientation and repositioning of chromosomes correlate with cell geometry-dependent gene expression. Mol. Biol. Cell 28, 1997–2009 (2017). This study demonstrates that altering the mechanical state of the cell induces chromosome repositioning and orientation to regulate gene expression.
Wang, Y., Ratna, P. & Shivashankar, G. V. Superresolution imaging of nanoscale chromosome contacts. Sci. Rep. 7, 42422 (2017).
Osborne, C. S. et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat. Genet. 36, 1065–1071 (2004). This study exemplifies the role of spatial gene clustering for co-regulation.
Noordermeer, D. et al. The dynamic architecture of Hox gene clusters. Science 334, 222–225 (2011).
Jin, F. et al. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature 503, 290–294 (2013).
Gialitakis, M., Arampatzi, P., Makatounakis, T. & Papamatheakis, J. Gamma interferon-dependent transcriptional memory via relocalization of a gene locus to PML nuclear bodies. Mol. Cell. Biol. 30, 2046–2056 (2010).
Fanucchi, S., Shibayama, Y., Burd, S., Weinberg, M. S. & Mhlanga, M. M. Chromosomal contact permits transcription between coregulated genes. Cell 155, 606–620 (2013).
Noordermeer, D. et al. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife 3, e02557 (2014).
Letsou, W. & Cai, L. Noncommutative biology: sequential regulation of complex networks. PLoS Comput. Biol 12, e1005089 (2016).
Thévenin, A. et al. Functional gene groups are concentrated within chromosomes, among chromosomes and in the nuclear space of the human genome. Nucleic Acids Res. 42, 9854–9861 (2014).
Zhu, Y. et al. Constructing 3D interaction maps from 1D epigenomes. Nat. Commun. 7, 10812 (2016).
Capurso, D., Bengtsson, H. & Segal, M. R. Discovering hotspots in functional genomic data superposed on 3D chromatin configuration reconstructions. Nucleic Acids Res. 44, 2028–2035 (2016).
Cisse, I. I. et al. Real-time dynamics of RNA polymerase II clustering in live human cells. Science 341, 664–667 (2013).
Virtanen, J. A. & Vartiainen, M. K. Diverse functions for different forms of nuclear actin. Curr. Opin. Cell Biol. 46, 33–38 (2017).
Spichal, M. & Fabre, E. The emerging role of the cytoskeleton in chromosome dynamics. Front. Genet. 8, 60 (2017).
Leight, J. L., Wozniak, M. A., Chen, S., Lynch, M. L. & Chen, C. S. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol. Biol. Cell 23, 781–791 (2012).
Bonev, B. & Cavalli, G. Organization and function of the 3D genome. Nat. Rev. Genet. 17, 661–678 (2016).
Sun, S. & Irvine, K. D. Cellular organization and cytoskeletal regulation of the Hippo signaling network. Trends Cell Biol. 26, 694–704 (2016).
Sun, S. C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017).
Schreiber, K. H. & Kennedy, B. K. When lamins go bad: nuclear structure and disease. Cell 152, 1365–1375 (2013).
Hatch, E. & Hetzer, M. Breaching the nuclear envelope in development and disease. J. Cell Biol. 205, 133–141 (2014).
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006).
Frost, B. Alzheimer's disease: an acquired neurodegenerative laminopathy. Nucleus 7, 275–283 (2016).
Zink, D., Fischer, A. H. & Nickerson, J. A. Nuclear structure in cancer cells. Nat. Rev. Cancer 4, 677–687 (2004).
Hnisz, D. et al. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science 351, 1454–1458 (2016).
Roukos, V. et al. Spatial dynamics of chromosome translocations in living cells. Science 341, 660–664 (2013).
Graham, D. M. & Burridge, K. Mechanotransduction and nuclear function. Curr. Opin. Cell Biol. 40, 98–105 (2016).
Miroshnikova, Y. A., Nava, M. M. & Wickström, S. A. Emerging roles of mechanical forces in chromatin regulation. J. Cell Sci. 130, 2243–2250 (2017).
McGregor, A. L., Hsia, C. R. & Lammerding, J. Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 40, 32–40 (2016).
Jorgens, D. M. et al. Deep nuclear invaginations are linked to cytoskeletal filaments — integrated bioimaging of epithelial cells in 3D culture. J. Cell Sci. 130, 177–189 (2017).
Harr, J. C., Gonzalez-Sandoval, A. & Gasser, S. M. Histones and histone modifications in perinuclear chromatin anchoring: from yeast to man. EMBO Rep. 17, 139–155 (2016).
Branco, M. R. & Pombo, A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 4, e138 (2006).
Skinner, B. M. & Johnson, E. E. Nuclear morphologies: their diversity and functional relevance. Chromosoma 126, 195–212 (2017).
Grys, B. T. et al. Machine learning and computer vision approaches for phenotypic profiling. J. Cell Biol. 216, 65–71 (2017).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Yu, K. et al. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat. Commun. 7, 12474 (2016).
Mahamid, J. et al. Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science 351, 969–972 (2016).
Li, G. & Reinberg, D. Chromatin higher-order structures and gene regulation. Curr. Opin. Genet. Dev. 21, 175–186 (2011).
Gonzalez-Sandoval, A. & Gasser, S. M. On TADs and LADs: spatial control over gene expression. Trends Genet. 32, 485–495 (2016).
Feuerborn, A. & Cook, P. R. Why the activity of a gene depends on its neighbors. Trends Genet. 31, 483–490 (2015).
Bolzer, A. et al. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 3, e157 (2005). This paper demonstrates that chromosomes are nonrandomly organized within the cell nucleus.
Bickmore, W. A. & van Steensel, B. Genome architecture: domain organization of interphase chromosomes. Cell 152, 1270–1284 (2013).
Iyer, K. V. et al. Modeling and experimental methods to probe the link between global transcription and spatial organization of chromosomes. PLoS ONE 7, e46628 (2012).
Cook, P. R. A model for all genomes: the role of transcription factories. J. Mol. Biol. 395, 1–10 (2010).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009). This study identifies genome-wide chromosome contacts by use of chromosome capture assays.
Rao, S. S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Chen, H. et al. Functional organization of the human 4D nucleome. Proc. Natl Acad. Sci. USA 112, 8002–8007 (2015).
Schmitt, A. D., Hu, M. & Ren, B. Genome-wide mapping and analysis of chromosome architecture. Nat. Rev. Mol. Cell Biol. 17, 743–755 (2016).
Norton, H. K. & Phillips Cremins, J. E. Crossed wires: 3D genome misfolding in human disease. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201611001 (2017).
Uhler, C. & Wright, S. J. Packing ellipsoids with overlap. SIAM Rev. 55, 671–706 (2013).
Boettiger, A. N. et al. Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature 529, 418–422 (2016).
Dong, B. et al. Super-resolution intrinsic fluorescence imaging of chromatin utilizing native, unmodified nucleic acids for contrast. Proc. Natl Acad. Sci. USA 113, 9716–9721 (2016).
Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013).
Ma, H. et al. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc. Natl Acad. Sci. USA 112, 3002–3007 (2015).
Ramani, V. et al. Massively multiplex single-cell Hi-C. Nat. Methods 14, 263–266 (2017).
Acknowledgements
C.U. was partially supported by the US Defense Advanced Research Projects Agency (DARPA) (W911NF-16-1-0551), the National Science Foundation (NSF) (1651995) and the US Office of Naval Research (N00014-17-1-2147). G.V.S. was funded by the Mechanobiology Institute, Singapore, the MOE-Tier 3 grant, Singapore, and the Italian Foundation for Cancer Research (FIRC) Institute of Molecular Oncology (IFOM), Milan, Italy. The authors thank members of the Uhler and Shivashankar laboratories for useful discussions.
Author information
Authors and Affiliations
Contributions
Both authors contributed to researching data for the article, discussion of the content and writing, reviewing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Transdifferentiation
-
The process in which a somatic cell is transformed into another type of somatic cell.
- Integrins
-
Cell surface transmembrane receptors involved in mechanosensing.
- Focal adhesion complexes
-
Cell membrane protein complexes that connect the actin cytoskeleton with the extracellular matrix.
- Cadherin
-
A transmembrane receptor that bridges cell–cell junctions.
- Actomyosin
-
Protein complexes comprising actin and myosin; they form contractile units.
- Junction proteins
-
Proteins that bridge the cytoskeleton of two neighbouring cells through the cell–cell junction.
- Magnetic twisting cytometry
-
A technique for applying precise forces to single cells.
- Visco-elastic coupling
-
The propensity of materials to exhibit viscous and elastic responses when deformed.
- Inverted formins
-
Actin-nucleating proteins located on the endoplasmic reticulum and other cellular organelles.
- G-actin
-
The globular form of monomeric actin.
- Entropic pressure
-
Forces generated by the intrinsic thermodynamic tendency to increase entropy.
- Progeria
-
A genetic disorder of premature ageing.
Rights and permissions
About this article
Cite this article
Uhler, C., Shivashankar, G. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol 18, 717–727 (2017). https://doi.org/10.1038/nrm.2017.101
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2017.101
This article is cited by
-
Histone H1.0 couples cellular mechanical behaviors to chromatin structure
Nature Cardiovascular Research (2024)
-
3D Genome Reconstruction from Partially Phased Hi-C Data
Bulletin of Mathematical Biology (2024)
-
A novel approach to risk exposure and epigenetics—the use of multidimensional context to gain insights into the early origins of cardiometabolic and neurocognitive health
BMC Medicine (2023)
-
Intracellular remodeling associated with endoplasmic reticulum stress modifies biomechanical compliance of bladder cells
Cell Communication and Signaling (2023)
-
Dynamic chromatin architectures provide insights into the genetics of cattle myogenesis
Journal of Animal Science and Biotechnology (2023)