Key Points
-
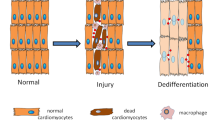
Mature, terminally differentiated cells have the capacity to de-differentiate or transdifferentiate in vivo.
-
De-differentiation and transdifferentiation can be forced experimentally, but these processes also occur physiologically in response to tissue injury and/or cell loss.
-
Cellular plasticity involves the repression of genes associated with the previous cell type, as well as activation of genes associated with the new cell type.
-
Cells may occupy 'intermediate' identity states while undergoing de-differentiation or transdifferentiation. Such changes can be reversible.
-
Cellular plasticity can be driven by factors that induce a new identity or by the loss of inhibitory factors that maintain the old identity.
Abstract
Biologists have long been intrigued by the possibility that cells can change their identity, a phenomenon known as cellular plasticity. The discovery that terminally differentiated cells can be experimentally coaxed to become pluripotent has invigorated the field, and recent studies have demonstrated that changes in cell identity are not limited to the laboratory. Specifically, certain adult cells retain the capacity to de-differentiate or transdifferentiate under physiological conditions, as part of an organ's normal injury response. Recent studies have highlighted the extent to which cell plasticity contributes to tissue homeostasis, findings that have implications for cell-based therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Holliday, R. Epigenetics: a historical overview. Epigenetics 1, 76–80 (2006).
Del Rio-Tsonis, K. & Tsonis, P. A. Eye regeneration at the molecular age. Dev. Dyn. 226, 211–224 (2003).
Gurdon, J. B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 10, 622–640 (1962).
Worley, M. I., Setiawan, L. & Hariharan, I. K. Regeneration and transdetermination in Drosophila imaginal discs. Annu. Rev. Genet. 46, 289–310 (2012).
Raff, M. Adult stem cell plasticity: fact or artifact? Annu. Rev. Cell Dev. Biol. 19, 1–22 (2003).
Jopling, C., Boue, S. & Izpisua Belmonte, J. C. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat. Rev. Mol. Cell Biol. 12, 79–89 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Tapscott, S. J. et al. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science 242, 405–411 (1988).
Davis, R. L., Weintraub, H. & Lassar, A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000 (1987).
Galliot, B. Hydra, a fruitful model system for 270 years. Int. J. Dev. Biol. 56, 411–423 (2012).
Baguna, J. The planarian neoblast: the rambling history of its origin and some current black boxes. Int. J. Dev. Biol. 56, 19–37 (2012).
Morgan, T. H. Growth and regeneration in Planaria lugubris. Arch. Ent. Mech. Org. 13, 179–212 (1902).
Goss, R. J. Kinetics of compensatory growth. Q. Rev. Biol. 40, 123–146 (1965).
de Cuevas, M. & Matunis, E. L. The stem cell niche: lessons from the Drosophila testis. Development 138, 2861–2869 (2011).
Tulina, N. & Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546–2549 (2001).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Brawley, C. & Matunis, E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304, 1331–1334 (2004). This paper demonstrates that differentiated spermatogonia can de-differentiate into germline stem cells in D. melanogaster mutants after extreme germline stem cell loss.
Sheng, X. R., Brawley, C. M. & Matunis, E. L. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell 5, 191–203 (2009).
Barroca, V. et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat. Cell Biol. 11, 190–196 (2009).
Nakagawa, T., Sharma, M., Nabeshima, Y., Braun, R. E. & Yoshida, S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328, 62–67 (2010).
Kai, T. & Spradling, A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 428, 564–569 (2004). This study shows that differentiating cyst cells in the D. melanogaster ovary can de-differentiate into germline stem cells when the germline stem cells are lost to excessive differentiation.
Steen, T. P. Stability of chondrocyte differentiation and contribution of muscle to cartilage during limb regeneration in the axolotl (Siredon mexicanum). J. Exp. Zool. 167, 49–78 (1968).
Tanaka, E. M. & Reddien, P. W. The cellular basis for animal regeneration. Dev. Cell 21, 172–185 (2011).
Kragl, M. et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460, 60–65 (2009).
Sandoval-Guzman, T. et al. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 14, 174–187 (2014). Sandoval-Guzman et al . find that after limb amputation, certain species of salamanders use de-differentiation of post-mitotic muscle fibres to produce more muscle progenitors for limb regeneration.
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
Kikuchi, K. et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464, 601–605 (2010).
Jopling, C. et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464, 606–609 (2010).
Blanpain, C. & Fuchs, E. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281 (2014).
Goodell, M. A., Nguyen, H. & Shroyer, N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat. Rev. Mol. Cell Biol. 16, 299–309 (2015).
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009).
Hsu, Y. C., Pasolli, H. A. & Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92–105 (2011).
Rompolas, P., Mesa, K. R. & Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518 (2013).
Page, M. E., Lombard, P., Ng, F., Gottgens, B. & Jensen, K. B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482 (2013).
Fullgrabe, A. et al. Dynamics of Lgr6 progenitor cells in the hair follicle, sebaceous gland, and interfollicular epidermis. Stem Cell Rep. 5, 843–855 (2015).
Seifert, A. W. et al. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561–565 (2012).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011).
van Es, J. H. et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 14, 1099–1104 (2012). This study finds that committed secretory precursors de-differentiate into new crypt stem cells following intestinal crypt injury, and that these de-differentiated stem cells can ultimately produce all of the cell types of the intestine.
Buczacki, S. J. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Stange, D. E. et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368 (2013).
Evans, M. J., Van Winkle, L. S., Fanucchi, M. V. & Plopper, C. G. Cellular and molecular characteristics of basal cells in airway epithelium. Exp. Lung Res. 27, 401–415 (2001).
Schoch, K. G. et al. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L631–L642 (2004).
Rock, J. R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106, 12771–12775 (2009).
Hong, K. U., Reynolds, S. D., Watkins, S., Fuchs, E. & Stripp, B. R. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L643–L649 (2004).
Tata, P. R. et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503, 218–223 (2013). This study demonstrates that differentiated cells are able to de-differentiate in response to stem cell ablation to replenish the stem cell population, and that this de-differentiation is likely to be inhibited and regulated by contact with existing stem cells.
Mirsky, R. et al. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J. Peripher. Nerv. Syst. 13, 122–135 (2008).
Painter, M. W. et al. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 83, 331–343 (2014).
Wang, H. et al. Turning terminally differentiated skeletal muscle cells into regenerative progenitors. Nat. Commun. 6, 7916 (2015).
Pajcini, K. V., Corbel, S. Y., Sage, J., Pomerantz, J. H. & Blau, H. M. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell 7, 198–213 (2010).
de Lau, W., Peng, W. C., Gros, P. & Clevers, H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 28, 305–316 (2014).
Monje, P. V., Soto, J., Bacallao, K. & Wood, P. M. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J. Biol. Chem. 285, 31024–31036 (2010).
Zhao, R. et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell 30, 151–165 (2014).
Wagers, A. J. & Weissman, I. L. Plasticity of adult stem cells. Cell 116, 639–648 (2004).
Huang, P. et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011).
Khurana, S. & Mukhopadhyay, A. In vitro transdifferentiation of adult hematopoietic stem cells: an alternative source of engraftable hepatocytes. J. Hepatol. 49, 998–1007 (2008).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Riddle, M. R., Weintraub, A., Nguyen, K. C., Hall, D. H. & Rothman, J. H. Transdifferentiation and remodeling of post-embryonic C. elegans cells by a single transcription factor. Development 140, 4844–4849 (2013).
Jarriault, S., Schwab, Y. & Greenwald, I. A. Caenorhabditis elegans model for epithelial-neuronal transdifferentiation. Proc. Natl Acad. Sci. USA 105, 3790–3795 (2008).
Freedman, B. D. et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell 26, 666–673 (2013).
Maki, N. et al. Expression of stem cell pluripotency factors during regeneration in newts. Dev. Dyn. 238, 1613–1616 (2009).
Maki, N. et al. Oocyte-type linker histone B4 is required for transdifferentiation of somatic cells in vivo. FASEB J. 24, 3462–3467 (2010).
Mizuno, N., Agata, K., Sawada, K., Mochii, M. & Eguchi, G. Expression of crystallin genes in embryonic and regenerating newt lenses. Dev. Growth Differ. 44, 251–256 (2002).
Reyer, R. W., Woolfitt, R. A. & Withersty, L. T. Stimulation of lens regeneration from the newt dorsal iris when implanted into the blastema of the regenerating limb. Dev. Biol. 32, 258–281 (1973).
Ito, M., Hayashi, T., Kuroiwa, A. & Okamoto, M. Lens formation by pigmented epithelial cell reaggregate from dorsal iris implanted into limb blastema in the adult newt. Dev. Growth Differ. 41, 429–440 (1999).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Song, K. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D. A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 (2008). This study finds that forced expression of β-cell-specific transcription factors in pancreatic acinar cells leads to transdifferentiation of acinar cells directly into functional β-cells.
Stanger, B. Z. Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol. 77, 179–200 (2015).
Yanger, K. et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 27, 719–724 (2013). Using genetic lineage tracing, Yanger et al . find that forced NOTCH signalling or injury is sufficient to induce hepatocytes to transdifferentiate into biliary cells in a stepwise process.
Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618 (2014).
Michalopoulos, G. K., Barua, L. & Bowen, W. C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 41, 535–544 (2005).
Koh, D. S., Cho, J. H. & Chen, L. Paracrine interactions within islets of Langerhans. J. Mol. Neurosci. 48, 429–440 (2012).
Cogger, K. & Nostro, M. C. Recent advances in cell replacement therapies for the treatment of type 1 diabetes. Endocrinology 156, 8–15 (2015).
Kushner, J. A., MacDonald, P. E. & Atkinson, M. A. Stem cells to insulin secreting cells: two steps forward and now a time to pause? Cell Stem Cell 15, 535–536 (2014).
Baeyens, L. et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat. Biotechnol. 32, 76–83 (2014).
Chen, Y. J. et al. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep. 6, 1046–1058 (2014).
Ferber, S. et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 6, 568–572 (2000).
Shternhall-Ron, K. et al. Ectopic PDX-1 expression in liver ameliorates type 1 diabetes. J. Autoimmun. 28, 134–142 (2007).
Horb, M. E., Shen, C. N., Tosh, D. & Slack, J. M. Experimental conversion of liver to pancreas. Curr. Biol. 13, 105–115 (2003).
Thorel, F. et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010). Thorel et al . determine that following β-cell ablation in the pancreas, α-cells can transdifferentiate into functional β-cells without needing exogenous factors to initiate transdifferentiation.
Chera, S. et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514, 503–507 (2014).
Gao, T. et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 19, 259–271 (2014).
Dhawan, S., Georgia, S., Tschen, S. I., Fan, G. & Bhushan, A. Pancreatic β cell identity is maintained by DNA methylation-mediated repression of Arx. Dev. Cell 20, 419–429 (2011).
Talchai, C., Xuan, S., Lin, H. V., Sussel, L. & Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 (2012).
Guo, S. et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 123, 3305–3316 (2013).
Zuryn, S. et al. Sequential histone-modifying activities determine the robustness of transdifferentiation. Science 345, 826–829 (2014).
Zong, Y. et al. Notch signaling controls liver development by regulating biliary differentiation. Development 136, 1727–1739 (2009).
Yanger, K. & Stanger, B. Z. Liver cell reprogramming: parallels with iPSC biology. Cell Cycle 13, 1211–1212 (2014).
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014).
Katsuyama, T. & Paro, R. Epigenetic reprogramming during tissue regeneration. FEBS Lett. 585, 1617–1624 (2011).
Slack, J. M. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat. Rev. Mol. Cell Biol. 8, 369–378 (2007).
Tosh, D. & Slack, J. M. How cells change their phenotype. Nat. Rev. Mol. Cell Biol. 3, 187–194 (2002).
Corbett, J. L. & Tosh, D. Conversion of one cell type into another: implications for understanding organ development, pathogenesis of cancer and generating cells for therapy. Biochem. Soc. Trans. 42, 609–616 (2014).
Shaheen, N. J. & Richter, J. E. Barrett's oesophagus. Lancet 373, 850–861 (2009).
Bhat, S. et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J. Natl Cancer Inst. 103, 1049–1057 (2011).
Hvid-Jensen, F., Pedersen, L., Drewes, A. M., Sorensen, H. T. & Funch-Jensen, P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N. Engl. J. Med. 365, 1375–1383 (2011).
Stanger, B. Z. & Hebrok, M. Control of cell identity in pancreas development and regeneration. Gastroenterology 144, 1170–1179 (2013).
Grippo, P. J., Nowlin, P. S., Demeure, M. J., Longnecker, D. S. & Sandgren, E. P. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 63, 2016–2019 (2003).
De La, O. J. et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl Acad. Sci. USA 105, 18907–18912 (2008).
Kopp, J. L. et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22, 737–750 (2012). This study shows that acinar-to-ductal metaplasia and expression of ductal genes are crucial for inducing acinar cells to give rise to pancreatic ductal adenocarcinoma, and suggests that cellular reprogramming may be a crucial step in tumour initiation.
Zhu, L., Shi, G., Schmidt, C. M., Hruban, R. H. & Konieczny, S. F. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am. J. Pathol. 171, 263–273 (2007).
Krah, N. M. et al. The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. eLife 4, e07125 (2015).
Fan, B. et al. Cholangiocarcinomas can originate from hepatocytes in mice. J. Clin. Invest. 122, 2911–2915 (2012).
Sekiya, S. & Suzuki, A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J. Clin. Invest. 122, 3914–3918 (2012).
Villanueva, A. et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology 143, 1660–1669.e7 (2012).
Li, H. et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 32, 38–47 (2012).
Abollo-Jimenez, F., Jimenez, R. & Cobaleda, C. Physiological cellular reprogramming and cancer. Semin. Cancer Biol. 20, 98–106 (2010).
Bjornson, C. R., Rietze, R. L., Reynolds, B. A., Magli, M. C. & Vescovi, A. L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283, 534–537 (1999).
Petersen, B. E. et al. Bone marrow as a potential source of hepatic oval cells. Science 284, 1168–1170 (1999).
Carriere, C., Seeley, E. S., Goetze, T., Longnecker, D. S. & Korc, M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc. Natl Acad. Sci. USA 104, 4437–4442 (2007).
Khan, M. S., Thornhill, J. A., Gaffney, E., Loftus, B. & Butler, M. R. Keratinising squamous metaplasia of the bladder: natural history and rationalization of management based on review of 54 years experience. Eur. Urol. 42, 469–474 (2002).
de Vries, A. C. & Kuipers, E. J. Epidemiology of premalignant gastric lesions: implications for the development of screening and surveillance strategies. Helicobacter 12 (Suppl. 2), 22–31 (2007).
Elson, D. A. et al. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 60, 1267–1275 (2000).
Daniels, J. M. & Sutedja, T. G. Detection and minimally invasive treatment of early squamous lung cancer. Ther. Adv. Med. Oncol. 5, 235–248 (2013).
Defourny, J. et al. Cochlear supporting cell transdifferentiation and integration into hair cell layers by inhibition of ephrin-B2 signalling. Nat. Commun. 6, 7017 (2015).
Jain, R. et al. Plasticity of Hopx+ type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 6, 6727 (2015).
Pfisterer, U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl Acad. Sci. USA 108, 10343–10348 (2011).
Nakagawa, N. & Duffield, J. S. Myofibroblasts in fibrotic kidneys. Curr. Pathobiol. Rep. 1, 189–198 (2013).
Tetteh, P. W. et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18, 203–213 (2016).
Acknowledgements
The authors are indebted to Pantelis Rompolas for helpful comments on the manuscript. A.M. is supported by a grant from the Cholangiocarcinoma Foundation. B.Z.S. is supported by grants from the US National Institutes of Health (NIH; DK104196 and CA169123), the Penn Institute for Regenerative Medicine, the Biesecker Pediatric Liver Center and the Abramson Family Cancer Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Progenitor cell
-
An immature cell, often lineage-restricted, that can proliferate and give rise to differentiated cells. This is often a short-term state compared to stem cell populations, which may be maintained for a lifetime. Progenitor cells are also sometimes referred to as transit-amplifying cells.
- Reprogramming
-
A change in the identity of a differentiated cell. Usage of this term often overlaps with de- and transdifferentiation, although reprogramming generally refers to a complete and stable shift. The most extreme example is reprogramming of a differentiated cell to a pluripotent state.
- Epimorphosis
-
Morgan's term for regeneration using cellular proliferation.
- Morphyllaxis
-
Morgan's term for regeneration using existing material in the animal, without relying on proliferation.
- Lineage tracing
-
Tracing the progeny and fate of a population of cells using permanent labelling.
- Schwann cells
-
Cells that surround and envelope neurons in myelin sheaths, allowing for proper conduction along the nerve.
- Lateral plate mesoderm
-
A developmental division of mesoderm that gives rise to tendon, bone, connective tissue and dermis within the vertebrate limb.
- Multinucleated myofibres
-
Syncytial muscle fibres formed from many muscle progenitors that fuse together to generate a single fibre with many nuclei.
- Satellite cells
-
PAX7+ muscle stem cells that reside next to muscle fibres and mediate muscle regeneration in many vertebrate species.
- Sarcomere apparatus
-
Actin, myosin and associated proteins within mature muscle fibres that are organized in such a way that they can move relative to each other to produce muscle contractions.
- Myelin
-
An electrically insulating sheath provided by Schwann cell membranes that surrounds axons.
- Linker histone
-
A histone that is responsible for stabilizing the complex of DNA wrapped around histones that forms nucleosomes.
- Somatic cell nuclear transfer
-
(SCNT). A technique whereby nuclei from differentiated cells are transplanted into oocytes. These nuclei are reprogrammed to a pluripotent state and can, ultimately, generate a new organism.
- Pluripotency factors
-
The OCT3/4, SOX2, KLF4 and MYC (OSKM) transcription factors that can induce differentiated cells to reprogramme into induced pluripotent stem cells. Also known as Yamanaka factors.
- Pancreatic islets of Langerhans
-
Endocrine cells in the pancreas that are responsible for producing the hormones used for glucose management.
- Glucagon
-
A hormone secreted by pancreatic α-cells that increases serum glucose levels.
- Somatostatin
-
A hormone secreted by pancreatic δ-cells that inhibits the secretion of other pancreatic hormones.
- Metaplasia
-
Changes in tissue whereby one cell type is replaced by another, often associated with increased cancer risk.
Rights and permissions
About this article
Cite this article
Merrell, A., Stanger, B. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol 17, 413–425 (2016). https://doi.org/10.1038/nrm.2016.24
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2016.24
This article is cited by
-
Homeostatic regulation of renewing tissue cell populations via crowding control: stability, robustness and quasi-dedifferentiation
Journal of Mathematical Biology (2024)
-
Targeting the key players of phenotypic plasticity in cancer cells by phytochemicals
Cancer and Metastasis Reviews (2024)
-
Regulation of chromatin organization during animal regeneration
Cell Regeneration (2023)
-
Genetic recording of in vivo cell proliferation by ProTracer
Nature Protocols (2023)
-
Reduced smooth muscle-fibroblasts transformation potentially decreases intestinal wound healing and colitis-associated cancer in ageing mice
Signal Transduction and Targeted Therapy (2023)