Key Points
-
The stomach is a versatile organ that protects against countless forms of endogenous and exogenous injury, mainly through the production of acid
-
The injury response of the stomach can be classified into two main patterns: one that protects against endogenous acid (superficial response) and one that adapts when the source of acid is lost or compromised (glandular response)
-
The glandular response is a process that is best understood in the gastric corpus and involves a replacement of injured epithelium with metaplastic cells, a process known as spasmolytic polypeptide-expressing metaplasia (SPEM)
-
Studies have highlighted the epithelial plasticity of the gastric corpus, in particular the ability of postmitotic zymogenic chief cells to re-enter the cell cycle and fuel the repair of injured epithelium
Abstract
Subjected to countless daily injuries, the stomach still functions as a remarkably efficient digestive organ and microbial filter. In this Review, we follow the lead of the earliest gastroenterologists who were fascinated by the antiseptic and digestive powers of gastric secretions. We propose that it is easiest to understand how the stomach responds to injury by stressing the central role of the most important gastric secretion, acid. The stomach follows two basic patterns of adaptation. The superficial response is a pattern whereby the surface epithelial cells migrate and rapidly proliferate to repair erosions induced by acid or other irritants. The stomach can also adapt through a glandular response when the source of acid is lost or compromised (that is, the process of oxyntic atrophy). We primarily review the mechanisms governing the glandular response, which is characterized by a metaplastic change in cellular differentiation known as spasmolytic polypeptide-expressing metaplasia (SPEM). We propose that the stomach, like other organs, exhibits marked cellular plasticity: the glandular response involves reprogramming mature cells to serve as auxiliary stem cells that replace lost cells. Unfortunately, such plasticity might mean that the gastric epithelium undergoes cycles of differentiation and de-differentiation that increase the risk of accumulating cancer-predisposing mutations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
De Fourcroy, A. F. in Élémens d'Histoire Naturelle et de Chimie 357–362 (Cuchet, Paris, 1791).
Spallanzani, L. Dissertations Relative to the Natural History of Animals and Vegetables Vol. 1 (J. Murray, London, 1789). This paper is one of the first treatises focusing on the stomach and its role in digestion.
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–386 (2015).
Colquhoun, A. et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 64, 1881–1888 (2015).
Carter, A. J. & Nguyen, C. N. A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Publ. Health 12, 526 (2012).
Bartle, H. J. & Harkins, M. J. The gastric secretion: its bactericidal value to man. Amer. J. Med. Sci. 169, 373–388 (1925).
Kim, T. H. & Shivdasani, R. A. Stomach development, stem cells and disease. Development 143, 554–565 (2016).
McCracken, K. W. et al. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature 541, 182–187 (2017). This paper is the first to produce mature lineages of gastric corpus epithelium from human embryonic stem cells and demonstrates how WNT has a role in gastric corpus development.
McCracken, K. W. & Wells, J. M. Mechanisms of embryonic stomach development. Semin. Cell Dev. Biol. 66, 36–42 (2017).
Willet, S. G. & Mills, J. C. Stomach Organ and Cell Lineage Differentiation: from Embryogenesis to Adult Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2, 546–559 (2016).
O'Connor, A. & O'Morain, C. Digestive function of the stomach. Dig. Dis. 32, 186–191 (2014).
Ban, S. in Morson and Dawson's Gastrointestinal Pathology (eds Shepherd, N. A. et al.) Ch. 9 (Wiley-Blackwell, Hoboken, 2013).
Kararli, T. T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16, 351–380 (1995).
De Bolos, C., Garrido, M. & Real, F. X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology 109, 723–734 (1995).
Longman, R. J. et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut 47, 792–800 (2000).
Hanby, A. M. et al. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology 105, 1110–1116 (1993).
Li, H. J. et al. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology 146, 754–764 (2014).
Watson, S. A., Grabowska, A. M., El-Zaatari, M. & Takhar, A. Gastrin — active participant or bystander in gastric carcinogenesis? Nat. Rev. Cancer 6, 936–946 (2006).
Choi, E. et al. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 63, 1711–1720 (2014).
Sato, A. Tuft cells. Anat. Sci. Int. 82, 187–199 (2007).
O'Neil, A., Petersen, C. P., Choi, E., Engevik, A. C. & Goldenring, J. R. Unique cellular lineage composition of the first gland of the mouse gastric corpus. J. Histochem. Cytochem. 65, 47–58 (2017).
Nam, K. T. et al. Gastric tumor development in Smad3-deficient mice initiates from forestomach/glandular transition zone along the lesser curvature. Lab. Invest. 92, 883–895 (2012).
Saqui-Salces, M. et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem. Cell Biol. 136, 191–204 (2011).
Zhang, Y. & Huang, X. Investigation of doublecortin and calcium/calmodulin-dependent protein kinase-like-1-expressing cells in the mouse stomach. J. Gastroenterol. Hepatol. 25, 576–582 (2010).
Prasanna, L. C. Analysis of the distribution of mucins in adult human gastric mucosa and its functional significance. J. Clin Diagn. Res. 10, AC01–AC04 (2016).
Ootani, A., Toda, S., Fujimoto, K. & Sugihara, H. Foveolar differentiation of mouse gastric mucosa in vitro. Am. J. Pathol. 162, 1905–1912 (2003).
Hellmig, S. et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J. Gastroenterol. Hepatol. 21, 1832–1838 (2006).
Leontiadis, G. I. et al. Effects of Helicobacter pylori infection on gastric emptying rate in patients with non-ulcer dyspepsia. World J. Gastroenterol. 10, 1750–1754 (2004).
Gannon, B., Browning, J., O'Brien, P. & Rogers, P. Mucosal microvascular architecture of the fundus and body of human stomach. Gastroenterology 86, 866–875 (1984).
Kvietys, P. R. in The Gastrointestinal Circulation Integrated Systems Physiology: From Molecule to Function (eds Granger, J. & Granger, D. N.) Ch. 9 (Morgan & Claypool Life Sciences, San Rafael, 2010).
Giannella, R. A., Broitman, S. A. & Zamcheck, N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 13, 251–256 (1972).
Rugge, M. et al. OLGA staging for gastritis: a tutorial. Dig. Liver Dis. 40, 650–658 (2008).
Dixon, M. F., Genta, R. M., Yardley, J. H. & Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20, 1161–1181 (1996).
Correa, P. Chronic gastritis: a clinico-pathological classification. Am. J. Gastroenterol. 83, 504–509 (1988).
Rugge, M. & Genta, R. M. Staging and grading of chronic gastritis. Hum. Pathol. 36, 228–233 (2005).
Schubert, M. L. Functional anatomy and physiology of gastric secretion. Curr. Opin. Gastroenterol. 31, 479–485 (2015).
Imhann, F. et al. Proton pump inhibitors affect the gut microbiome. Gut 65, 740–748 (2016).
Sanduleanu, S., Jonkers, D., de Bruine, A., Hameeteman, W. & Stockbrugger, R. W. Changes in gastric mucosa and luminal environment during acid-suppressive therapy: a review in depth. Dig. Liver Dis. 33, 707–719 (2001).
McDonald, E. G., Milligan, J., Frenette, C. & Lee, T. C. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern. Med. 175, 784–791 (2015).
Leonard, J., Marshall, J. K. & Moayyedi, P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am. J. Gastroenterol. 102, 2047–2056 (2007).
Janarthanan, S., Ditah, I., Adler, D. G. & Ehrinpreis, M. N. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am. J. Gastroenterol. 107, 1001–1010 (2012).
Freedberg, D. E., Lebwohl, B. & Abrams, J. A. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin. Lab Med. 34, 771–785 (2014).
Tielleman, T., Bujanda, D. & Cryer, B. Epidemiology and risk factors for upper gastrointestinal bleeding. Gastrointest. Endosc. Clin. N. Am. 25, 415–428 (2015).
Lanas, A. & Chan, F. K. Peptic ulcer disease. Lancet 16, 32404–32407 (2017).
Souza, R. F. et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137, 1776–1784 (2009).
Taylor, W. H. Pepsins of patients with peptic ulcer. Nature 227, 76–77 (1970).
Allen, A. & Flemstrom, G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 288, C1–19 (2005).
Wallace, J. L. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol. Rev. 88, 1547–1565 (2008).
Dixon, M. F., O'Connor, H. J., Axon, A. T., King, R. F. & Johnston, D. Reflux gastritis: distinct histopathological entity? J. Clin. Pathol. 39, 524–530 (1986).
Singer, M. V., Leffmann, C., Eysselein, V. E., Calden, H. & Goebell, H. Action of ethanol and some alcoholic beverages on gastric acid secretion and release of gastrin in humans. Gastroenterology 93, 1247–1254 (1987).
Boltin, D. & Niv, Y. Pharmacological and alimentary alteration of the gastric barrier. Best Pract. Res. Clin. Gastroenterol. 28, 981–994 (2014).
Ma, L., Chow, J. Y. & Cho, C. H. Effects of cigarette smoking on gastric ulcer formation and healing: possible mechanisms of action. J. Clin. Gastroenterol. 27 (Suppl. 1), S80–S86 (1998).
Ichikawa, T. I. K., in Protective Effects of Gastric Mucus, Gastritis, and Gastric Cancer — New Insights in Gastroprotection, Diagnosis, and Treatments (ed. Tonino, P.) Ch. 1 (InTech, Rijeka, 2011).
Allen, A., Flemstrom, G., Garner, A. & Kivilaakso, E. Gastroduodenal mucosal protection. Physiol. Rev. 73, 823–857 (1993).
McColl, K. E. The elegance of the gastric mucosal barrier: designed by nature for nature. Gut 61, 787–788 (2012).
Yandrapu, H. & Sarosiek, J. Protective factors of the gastric and duodenal mucosa: an overview. Curr. Gastroenterol. Rep. 17, 24 (2015).
Ho, S. B. et al. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig. Dis. Sci. 49, 1598–1606 (2004).
Thim, L., Madsen, F. & Poulsen, S. S. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Invest. 32, 519–527 (2002).
Ikezawa, T. et al. Appearance of specific mucins recognized by monoclonal antibodies in rat gastric mucosa healing from HCl-induced gastric mucosal damage. J. Gastroenterol. 39, 113–119 (2004).
Hayashida, H. et al. Expression of a specific mucin type recognized by monoclonal antibodies in the rat gastric mucosa regenerating from acetic acid-induced ulcer. Scand. J. Gastroenterol. 36, 467–473 (2001).
Boltin, D. et al. Gastric mucin expression in Helicobacter pylori-related, nonsteroidal anti-inflammatory drug-related and idiopathic ulcers. World J. Gastroenterol. 18, 4597–4603 (2012).
Robert, A., Nezamis, J. E., Lancaster, C. & Hanchar, A. J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 77, 433–443 (1979). This study is one of the first to elucidate the protective roles of prostaglandins in the superficial response to gastric irritants.
Konturek, S. J. et al. Role of locally generated prostaglandins in adaptive gastric cytoprotection. Dig. Dis. Sci. 27, 967–971 (1982).
Peskar, B. M. Role of cyclooxygenase isoforms in gastric mucosal defence. J. Physiol. Paris 95, 3–9 (2001).
Ham, M., Akiba, Y., Takeuchi, K., Montrose, M. H. & Kaunitz, J. D. in Physiology of the Gastrointestinal Tract (ed. Johnson, L. R.) 1169–1208 (Academic Press, Oxford, 2012).
Ohnishi, A. et al. EP1 and EP4 receptors mediate exocytosis evoked by prostaglandin E(2) in guinea-pig antral mucous cells. Exp. Physiol. 86, 451–460 (2001).
Starodub, O. T., Demitrack, E. S., Baumgartner, H. K. & Montrose, M. H. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am. J. Physiol. Cell Physiol. 294, C223–C232 (2008).
Melcarne, L., Garcia-Iglesias, P. & Calvet, X. Management of NSAID-associated peptic ulcer disease. Expert Rev. Gastroenterol. Hepatol. 10, 723–733 (2016).
Scarpignato, C. & Pelosini, I. Prevention and treatment of non-steroidal anti-inflammatory drug-induced gastro-duodenal damage: rationale for the use of antisecretory compounds. Ital. J. Gastroenterol. Hepatol. 31 (Suppl. 1), S63–S72 (1999).
Takeuchi, K. Pathogenesis of NSAID-induced gastric damage: importance of cyclooxygenase inhibition and gastric hypermotility. World J. Gastroenterol. 18, 2147–2160 (2012).
Tarnawski, A. S., Ahluwalia, A. & Jones, M. K. The mechanisms of gastric mucosal injury: focus on microvascular endothelium as a key target. Curr. Med. Chem. 19, 4–15 (2012).
Capoccia, B. J., Huh, W. J. & Mills, J. C. How form follows functional genomics: gene expression profiling gastric epithelial cells with a particular discourse on the parietal cell. Physiol. Genom. 37, 67–78 (2009).
Lo, H. G. et al. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev. 31, 154–171 (2017).
Fennerty, M. B. Pathophysiology of the upper gastrointestinal tract in the critically ill patient: rationale for the therapeutic benefits of acid suppression. Crit. Care Med. 30, S351–355 (2002).
Spirt, M. J. Stress-related mucosal disease. Curr. Treat. Opt. Gastroenterol. 6, 135–145 (2003).
Pilkington, K. B., Wagstaff, M. J. & Greenwood, J. E. Prevention of gastrointestinal bleeding due to stress ulceration: a review of current literature. Anaesth. Intensive Care 40, 253–259 (2012).
Paimela, H. et al. Restitution of frog gastric mucosa in vitro: effect of basic fibroblast growth factor. Gastroenterology 104, 1337–1345 (1993).
Kawano, S. & Tsuji, S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J. Gastroenterol. Hepatol. 15 (Suppl.), D1–D6 (2000).
Jones, M. K. et al. Activation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injury. Am. J. Physiol. 276, G1345–G1355 (1999).
Szabo, S. “Gastric cytoprotection” is still relevant. J. Gastroenterol. Hepatol. 29 (Suppl. 4), 124–132 (2014).
Hernandez, L. A. et al. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am. J. Physiol. 253, H699–703 (1987).
Pique, J. M., Whittle, B. J. & Esplugues, J. V. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur. J. Pharmacol. 174, 293–296 (1989).
Brown, J. F., Keates, A. C., Hanson, P. J. & Whittle, B. J. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 265, G418–G422 (1993).
Brown, J. F., Hanson, P. J. & Whittle, B. J. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur. J. Pharmacol. 223, 103–104 (1992).
Kato, S., Kitamura, M., Korolkiewicz, R. P. & Takeuchi, K. Role of nitric oxide in regulation of gastric acid secretion in rats: effects of NO donors and NO synthase inhibitor. Br. J. Pharmacol. 123, 839–846 (1998).
Wallace, J. L. & Miller, M. J. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology 119, 512–520 (2000).
Takeuchi, K., Yasuhiro, T., Asada, Y. & Sugawa, Y. Role of nitric oxide in pathogenesis of aspirin-induced gastric mucosal damage in rats. Digestion 59, 298–307 (1998).
Jimenez, D. et al. Mechanisms involved in protection afforded by L-arginine in ibuprofen-induced gastric damage: role of nitric oxide and prostaglandins. Dig. Dis. Sci. 47, 44–53 (2002).
Wallace, J. L. et al. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology 107, 173–179 (1994).
Kodela, R., Chattopadhyay, M., Velazquez-Martinez, C. A. & Kashfi, K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 98, 564–572 (2015).
Lanas, A. et al. Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N. Engl. J. Med. 343, 834–839 (2000).
Lanas, A. et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am. J. Gastroenterol. 102, 507–515 (2007).
Sorbye, H., Svanes, C., Stangeland, L., Kvinnsland, S. & Svanes, K. Epithelial restitution and cellular proliferation after gastric mucosal damage caused by hypertonic NaCl in rats. Virchows Arch. A Pathol. Anat. Histopathol 413, 445–455 (1988).
Lacy, E. R. & Ito, S. Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab. Invest. 51, 573–583 (1984).
Karam, S. M. & Leblond, C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat. Rec. 236, 280–296 (1993). This is a landmark study that qualitatively and quantitatively illustrates the dynamics of gastric stem cells giving rise to the pit cell lineage.
Mimuro, H. et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe 2, 250–263 (2007).
Reinhart, W. H., Muller, O. & Halter, F. Influence of long-term 16,16-dimethyl prostaglandin E2 treatment on the rat gastrointestinal mucosa. Gastroenterology 85, 1003–1010 (1983).
Khurana, S. S. et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J. Biol. Chem. 288, 16085–16097 (2013).
Rokutan, K., Yamada, M., Torigoe, J. & Saito, T. Transforming growth factor-beta inhibits proliferation and maturation of cultured guinea pig gastric pit cells. Am. J. Physiol. 275, G526–G533 (1998).
Abe, S. et al. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig. Dis. Sci. 42, 1199–1209 (1997).
Nam, K. T. et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology 136, 1288–1296 (2009).
Murayama, Y. et al. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology 109, 1051–1059 (1995).
Coffey, R. J., Romano, M., Polk, W. H. & Dempsey, P. J. Roles for transforming growth factor-alpha in gastric physiology and pathophysiology. Yale J. Biol. Med. 65, 693–704 (1992).
Jones, M. K., Tomikawa, M., Mohajer, B. & Tarnawski, A. S. Gastrointestinal mucosal regeneration: role of growth factors. Front. Biosci. 4, D303–D309 (1999).
Polk, W. H. Jr. et al. Increased production of transforming growth factor alpha following acute gastric injury. Gastroenterology 102, 1467–1474 (1992).
Pai, R. & Tarnawski, A. Signal transduction cascades triggered by EGF receptor activation: relevance to gastric injury repair and ulcer healing. Dig. Dis. Sci. 43, 14S–22S (1998).
Poulsen, S. S. On the role of epidermal growth factor in the defence of the gastroduodenal mucosa. Scand. J. Gastroenterol. Suppl. 128, 20–23 (1987).
Goldenring, J. R. et al. Overexpression of transforming growth factor-alpha alters differentiation of gastric cell lineages. Dig. Dis. Sci. 41, 773–784 (1996).
Takagi, H., Jhappan, C., Sharp, R. & Merlino, G. Hypertrophic gastropathy resembling Menetrier's disease in transgenic mice overexpressing transforming growth factor alpha in the stomach. J. Clin. Invest. 90, 1161–1167 (1992).
Coffey, R. J., Washington, M. K., Corless, C. L. & Heinrich, M. C. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J. Clin. Invest. 117, 70–80 (2007).
Genta, R. M. Review article: Gastric atrophy and atrophic gastritis — nebulous concepts in search of a definition. Aliment. Pharmacol. Ther. 12 (Suppl. 1), 17–23 (1998).
Goldenring, J. R. & Nam, K. T. Oxyntic atrophy, metaplasia, and gastric cancer. Prog. Mol. Biol. Transl Sci. 96, 117–131 (2010).
Weis, V. G. & Goldenring, J. R. Current understanding of SPEM and its standing in the preneoplastic process. Gastr. Cancer 12, 189–197 (2009).
Petersen, C. P., Mills, J. C. & Goldenring, J. R. Murine models of gastric corpus preneoplasia. Cell. Mol. Gastroenterol. Hepatol. 3, 11–26 (2017).
Goldenring, J. R., Nam, K. T. & Mills, J. C. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp. Cell Res. 317, 2759–2764 (2011).
Schmidt, P. H. et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab. Invest. 79, 639–646 (1999). This paper is the first to identify a metaplastic lineage, termed spasmolytic polypeptide-expressing metaplasia (SPEM), from gastric corpus biopsy samples of patients chronically infected with Helicobacter pylori.
Lauwers, G. Y. in Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas (eds Odze, R. D. & Goldblum, J. R.) Ch. 23 (Saunders/Elsevier, Amsterdam, 2009).
Saenz, J. B., Burclaff, J. & Mills, J. C. Modeling murine gastric metaplasia through tamoxifen-induced acute parietal cell loss. Methods Mol. Biol. 1422, 329–339 (2016).
Nomura, S. et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G362–G375 (2005).
Huh, W. J. et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142, 21–24 (2012).
Burclaff, J., Osaki, L. H., Liu, D., Goldenring, J. R. & Mills, J. C. Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology 152, 762–766 (2017).
Demitrack, E. S. et al. NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G133–G144 (2017). This group had previously demonstrated the role of NOTCH1 and NOTCH2 in the gastric antrum and here show that NOTCH1 and NOTCH2 signalling promotes stem and progenitor cell proliferation in the gastric corpus.
Shiotani, A. et al. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am. J. Gastroenterol. 100, 581–587 (2005).
Merchant, J. L. & Ding, L. Hedgehog signaling links chronic inflammation to gastric cancer precursor lesions. Cell. Mol. Gastroenterol. Hepatol. 3, 201–210 (2017). This review nicely summarizes the role of Hedgehog signalling in promoting a metaplastic milieu.
Fox, J. G. et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology 124, 1879–1890 (2003).
Zavros, Y. et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene 24, 2354–2366 (2005).
Todisco, A. Regulation of gastric metaplasia, dysplasia, and neoplasia by bone morphogenetic protein signaling. Cell. Mol. Gastroenterol. Hepatol. 3, 339–347 (2017). This paper shows that transgenic expression of the bone morphogenetic protein inhibitor Noggin results in gastric epithelial changes consistent with SPEM, implicating a role for BMP signalling in gastric epithelial homeostasis.
Shinohara, M. et al. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology 139, 2050–2060 (2010).
Syu, L. J. et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am. J. Pathol. 181, 2114–2125 (2012).
Serizawa, T. et al. Gastric metaplasia induced by Helicobacter pylori is associated with enhanced SOX9 expression via interleukin-1 signaling. Infect. Immun. 84, 562–572 (2015).
Waghray, M. et al. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology 138, 562–572 (2010).
Buzzelli, J. N. et al. IL33 is a stomach Alarmin that initiates a skewed Th2 response to injury and infection. Cell. Mol. Gastroenterol. Hepatol. 1, 203–221 (2015).
Petersen, C. P. et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut https://doi.org/10.1136/gutjnl-2016-312779 (2017).
Rugge, M., Genta, R. M. & Group, O. Staging gastritis: an international proposal. Gastroenterology 129, 1807–1808 (2005).
Sepulveda, A. R. & Patil, M. Practical approach to the pathologic diagnosis of gastritis. Arch. Pathol. Lab Med. 132, 1586–1593 (2008).
Miftahussurur, M., Yamaoka, Y. & Graham, D. Y. Helicobacter pylori as an oncogenic pathogen, revisited. Expert Rev. Mol. Med. 19, e4 (2017).
Rugge, M. et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 56, 631–636 (2007).
Gjeorgjievski, M. & Cappell, M. S. Portal hypertensive gastropathy: a systematic review of the pathophysiology, clinical presentation, natural history and therapy. World J. Hepatol. 8, 231–262 (2016).
Komorowski, R. A. & Caya, J. G. Hyperplastic gastropathy. Clinicopathologic correlation. Am. J. Surg. Pathol. 15, 577–585 (1991).
Gun, F., Abbasoglu, L. & Celik, A. Acute gastric perforation after acid ingestion. J. Pediatr. Gastroenterol. Nutr. 35, 360–362 (2002).
Ovenden, C. et al. Occult upper gastrointestinal mucosal abnormalities in critically ill patients. Acta Anaesthesiol. Scand. 61, 216–223 (2017).
Andersen, L. P. Colonization and infection by Helicobacter pylori in humans. Helicobacter 12 (Suppl. 2), 12–15 (2007).
Sugano, K. et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64, 1353–1367 (2015).
Goldenring, J. R., Nam, K. T., Wang, T. C., Mills, J. C. & Wright, N. A. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 138, 2207–2210 (2010).
Ianiro, G., Molina-Infante, J. & Gasbarrini, A. Gastric microbiota. Helicobacter 20 (Suppl. 1), 68–71 (2015).
Neumann, W. L., Coss, E., Rugge, M. & Genta, R. M. Autoimmune atrophic gastritis — pathogenesis, pathology and management. Nat. Rev. Gastroenterol. Hepatol. 10, 529–541 (2013). This Review concisely and comprehensively summarizes the clinical, endoscopic and histopathological aspects of autoimmune gastritis.
Claeys, D., Faller, G., Appelmelk, B. J., Negrini, R. & Kirchner, T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 115, 340–347 (1998).
Negrini, R. et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology 111, 655–665 (1996).
Appelmelk, B. J., Faller, G., Claeys, D., Kirchner, T. & Vandenbroucke-Grauls, C. M. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol. Today 19, 296–299 (1998).
Bergman, M. P. et al. The story so far: Helicobacter pylori and gastric autoimmunity. Int. Rev. Immunol. 24, 63–91 (2005).
Hooi, J. K. Y. et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153, 420–429 (2017).
Ernst, P. B., Peura, D. A. & Crowe, S. E. The translation of Helicobacter pylori basic research to patient care. Gastroenterology 130, 188–206 (2006).
Ernst, P. B. & Gold, B. D. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54, 615–640 (2000).
NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA 272, 65–69 (1994).
Kuipers, E. J., Thijs, J. C. & Festen, H. P. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment. Pharmacol. Ther. 9 (Suppl. 2), 59–69 (1995).
Malfertheiner, P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig. Dis. 29, 459–464 (2011).
Bertaux-Skeirik, N. et al. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J. Pathol. 242, 463–475 (2017).
Kuipers, E. J. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment. Pharmacol. Ther. 13 (Suppl. 1), 3–11 (1999).
Zhang, Y. et al. Clinical significance of spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia in EBV-associated and EBV-negative gastric cancer. Hum. Pathol. 63, 128–138 (2017).
Hansson, L. E. et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 335, 242–249 (1996).
Graham, D. Y. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J. Gastroenterol. 20, 5191–5204 (2014).
Graham, D. Y. & Asaka, M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J. Gastroenterol. 45, 1–8 (2010).
Watabe, H. et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 54, 764–768 (2005).
Gifford, G. B. et al. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut 66, 1001–1011 (2017).
Merrell, A. J. & Stanger, B. Z. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 17, 413–425 (2016).
Burclaff, J. & Mills, J. C. Cell biology: healthy skin rejects cancer. Nature 548, 289–290 (2017).
Mills, J. C. & Sansom, O. J. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci. Signal. 8, re8 (2015).
Demitrack, E. S. & Samuelson, L. C. Notch as a driver of gastric epithelial cell proliferation. Cell. Mol. Gastroenterol. Hepatol. 3, 323–330 (2017).
Hayakawa, Y., Fox, J. G. & Wang, T. C. The origins of gastric cancer from gastric stem cells: lessons from mouse models. Cell. Mol. Gastroenterol. Hepatol. 3, 331–338 (2017).
Karam, S. M. & Leblond, C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat. Rec. 236, 259–279 (1993). The morphological characteristics of the isthmal stem cell are described in this pivotal ultrastructural analysis.
Lee, E. R. & Leblond, C. P. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am. J. Anat. 172, 205–224 (1985).
Karam, S. M. & Leblond, C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat. Rec. 236, 297–313 (1993). This is a landmark study that describes the morphological characteristics of the epithelial lineages from the neck and base regions of the gastric corpus unit as well as the dynamics of epithelial differentiation in the corpus gland.
Ramsey, V. G. et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134, 211–222 (2007).
Bjerknes, M. & Cheng, H. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G767–G777 (2002).
Barker, N. et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010). This paper describes one of the first lineage tracing experiments in the stomach and identifies LGR5 as an antral stem cell marker.
Hayakawa, Y. et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut 64, 544–553 (2015).
Sigal, M. et al. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology 148, 1392–1404 (2015).
Leushacke, M. et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat. Cell Biol. 19, 774–786 (2017). This study demonstrates that Lgr5 -expressing chief cells are recruited to fuel epithelial renewal following glandular injury, suggesting that chief cells can acquire stemness.
Lee, E. R. & Leblond, C. P. Dynamic histology of the antral epithelium in the mouse stomach: IV. Ultrastructure and renewal of gland cells. Am. J. Anat. 172, 241–259 (1985). This study represents the first systematic analysis of stem cell dynamics and differentiation in the gastric antrum.
Lee, E. R. Dynamic histology of the antral epithelium in the mouse stomach: III. Ultrastructure and renewal of pit cells. Am. J. Anat. 172, 225–240 (1985).
Hayakawa, Y. et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28, 800–814 (2015).
Kretzschmar, K. & Watt, F. M. Lineage tracing. Cell 148, 33–45 (2012).
Li, N., Nakauka-Ddamba, A., Tobias, J., Jensen, S. T. & Lengner, C. J. Mouse label-retaining cells are molecularly and functionally distinct from reserve intestinal stem cells. Gastroenterology 151, 298–310 (2016).
Qiao, X. T. et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology 133, 1989–1998 (2007).
Arnold, K. et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317–329 (2011).
Tsukamoto, T. et al. Down-regulation of a gastric transcription factor, Sox2, and ectopic expression of intestinal homeobox genes, Cdx1 and Cdx2: inverse correlation during progression from gastric/intestinal-mixed to complete intestinal metaplasia. J. Cancer Res. Clin. Oncol. 130, 135–145 (2004).
Sigal, M. et al. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 548, 451–455 (2017). This study identifies Axin 2 as a marker of isthmal stem cells in the antrum and highlights the relative potential of various cell populations along the antral unit to serve as stem cells.
Dedhia, P. H., Bertaux-Skeirik, N., Zavros, Y. & Spence, J. R. Organoid models of human gastrointestinal development and disease. Gastroenterology 150, 1098–1112 (2016).
Schlaermann, P. et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 65, 202–213 (2016).
Bartfeld, S. et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136 (2015).
Fatehullah, A., Tan, S. H. & Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254 (2016).
Shimizu, T. et al. Characterization of progressive metaplasia in the gastric corpus mucosa of Mongolian gerbils infected with Helicobacter pylori. J. Pathol. 239, 399–410 (2016).
Jeong, S. et al. Distinct metaplastic and inflammatory phenotypes in autoimmune and adenocarcinoma-associated chronic atrophic gastritis. United Eur. Gastroenterol. J. 5, 37–44 (2017).
Nguyen, T. L. et al. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res. 73, 2117–2126 (2013).
Hayakawa, Y., Fox, J. G. & Wang, T. C. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell. Mol. Gastroenterol. Hepatol. 4, 89–94 (2017).
Nam, K. T. et al. Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut 61, 1678–1685 (2012).
Nam, K. T. et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139, 2028–2037 (2010). The authors of this study demonstrate that metaplastic cells lineage trace from chief cells following acute or chronic glandular injury, suggesting that mature chief cells are the source of metaplasia.
Choi, E., Hendley, A. M., Bailey, J. M., Leach, S. D. & Goldenring, J. R. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology 150, 918–930 (2016).
Matsuo, J. et al. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology 152, 218–231 (2017).
Stange, D. E. et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368 (2013). This study identifies Troy as a stem cell marker expressed by mature chief cells at the base of corpus units and highlights the plasticity of chief cells under certain forms of glandular injury.
Capoccia, B. J. et al. The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. J. Clin. Invest. 123, 1475–1491 (2013).
Radyk, M. D. & Mills, J. C. A chief source of cancer and repair in stomachs. EMBO J. 36, 2318–2320 (2017).
Lennerz, J. K. et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am. J. Pathol. 177, 1514–1533 (2010).
Radyk, M., Burclaff, J., Willet, S. G. & Mills, J. C. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology (in press).
Mills, J. C. & Goldenring, J. R. Metaplasia in the stomach arises from gastric chief cells. Cell. Mol. Gastroenterol. Hepatol. 4, 85–88 (2017).
Raven, A. et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 547, 350–354 (2017).
Iovanna, J. L., Lechene de la Porte, P. & Dagorn, J. C. Expression of genes associated with dedifferentiation and cell proliferation during pancreatic regeneration following acute pancreatitis. Pancreas 7, 712–718 (1992).
Jensen, J. N. et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128, 728–741 (2005).
Pinho, A. V. et al. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut 60, 958–966 (2011).
Storz, P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 14, 296–304 (2017).
Weis, V. G. et al. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G67–G76 (2017).
Rubio, C. A., Jaramillo, E., Suzuki, G., Lagergren, P. & Nesi, G. Antralization of the gastric mucosa of the incisura angularis and its gastrin expression. Int. J. Clin. Exp. Pathol. 2, 65–70 (2009).
Xia, H. H. et al. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): a link between Helicobacter pylori infection and intestinal metaplasia? Am. J. Gastroenterol. 95, 114–121 (2000).
Xia, H. H. et al. Topographic association of gastric epithelial expression of Ki-67, Bax, and Bcl-2 with antralization in the gastric incisura, body, and fundus. Am. J. Gastroenterol. 97, 3023–3031 (2002).
Xia, H. H. et al. Antralization of gastric incisura is topographically associated with increased gastric epithelial apoptosis and proliferation, but not with CagA seropositivity. J. Gastroenterol. Hepatol. 19, 1257–1263 (2004).
Genta, R. M. & Rugge, M. Review article: pre-neoplastic states of the gastric mucosa — a practical approach for the perplexed clinician. Aliment. Pharmacol. Ther. 15 (Suppl. 1), 43–50 (2001).
Genta, R. M. Atrophy and atrophic gastritis: one step beyond the Sydney system. Ital. J. Gastroenterol. Hepatol. 30 (Suppl. 3), S273–275 (1998).
Rogers, A. B. & Houghton, J. Helicobacter-based mouse models of digestive system carcinogenesis. Methods Mol. Biol. 511, 267–295 (2009).
Wright, N. A. Migration of the ductular elements of gut-associated glands gives clues to the histogenesis of structures associated with responses to acid hypersecretory state: the origins of “gastric metaplasia” in the duodenum of the specialized mucosa of barrett's esophagus and of pseudopyloric metaplasia. Yale J. Biol. Med. 69, 147–153 (1996).
Nozaki, K. et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 134, 511–522 (2008).
El-Zimaity, H. M. Gastric atrophy, diagnosing and staging. World J. Gastroenterol. 12, 5757–5762 (2006).
Van Zanten, S. J., Dixon, M. F. & Lee, A. The gastric transitional zones: neglected links between gastroduodenal pathology and Helicobacter ecology. Gastroenterology 116, 1217–1229 (1999).
Stave, R., Brandtzaeg, P., Nygaard, K. & Fausa, O. The transitional body-antrum zone in resected human stomachs. Anatomical outline and parietal-cell and gastrin-cell characteristics in peptic ulcer disease. Scand. J. Gastroenterol. 13, 685–691 (1978).
Peterson, W. L. Review article: Helicobacter pylori and gastric adenocarcinoma. Aliment. Pharmacol. Ther. 16 (Suppl. 1), 40–46 (2002).
Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe 15, 306–316 (2014).
Tsugawa, H. et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe 12, 764–777 (2012).
Greenfield, L. K. & Jones, N. L. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 21, 602–612 (2013).
Panella, C. et al. Proliferative activity of gastric epithelium in progressive stages of Helicobacter pylori infection. Dig. Dis. Sci. 41, 1132–1138 (1996).
Oh, J. D., Kling-Backhed, H., Giannakis, M., Engstrand, L. G. & Gordon, J. I. Interactions between gastric epithelial stem cells and Helicobacter pylori in the setting of chronic atrophic gastritis. Curr. Opin. Microbiol. 9, 21–27 (2006).
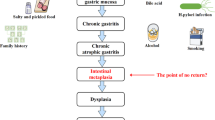
Correa, P. & Piazuelo, M. B. The gastric precancerous cascade. J. Dig. Dis. 13, 2–9 (2012).
Camargo, M. C. et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut 60, 1644–1649 (2011).
You, W. C. et al. Comparison of the anatomic distribution of stomach cancer and precancerous gastric lesions. Jpn J. Cancer Res. 83, 1150–1153 (1992).
Wanebo, H. J. et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann. Surg. 218, 583–592 (1993).
Guerra, C. et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302 (2007).
Collins, M. A. et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Invest. 122, 639–653 (2012).
Huang, H. et al. Oncogenic K-Ras requires activation for enhanced activity. Oncogene 33, 532–535 (2014).
Cassaro, M. et al. Topographic patterns of intestinal metaplasia and gastric cancer. Am. J. Gastroenterol. 95, 1431–1438 (2000). This study examines the extent and distribution of intestinal metaplasia in Colombian individuals with and without gastric cancer and finds that certain patterns of gastric intestinal metaplasia correlate with an increased risk of developing gastric cancer.
Odze, R. D. Unraveling the mystery of the gastroesophageal junction: a pathologist's perspective. Am. J. Gastroenterol. 100, 1853–1867 (2005).
Chandrasoma, P. RE: Odze RD. unraveling the mystery of the gastroesophageal junction: a pathologist's perspective. Am. J. Gastroenterol. 101, 199 (2006).
Derdoy, J. J. et al. The gastric cardia: to be or not to be? Am. J. Surg. Pathol. 27, 499–504 (2003).
Buas, M. F. & Vaughan, T. L. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin. Radiat. Oncol. 23, 3–9 (2013).
Cancer Genome Atlas Research, N. et al. Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017).
Odze, R. D. Pathology of the gastroesophageal junction. Semin. Diagn. Pathol. 22, 256–265 (2005).
Souza, R. F., Krishnan, K. & Spechler, S. J. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G211–G218 (2008).
Chandrasoma, P. Controversies of the cardiac mucosa and Barrett's oesophagus. Histopathology 46, 361–373 (2005).
Lavery, D. L. et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett's epithelium, replicates pyloric-type gastric glands. Gut 63, 1854–1863 (2014).
McDonald, S. A., Lavery, D., Wright, N. A. & Jansen, M. Barrett oesophagus: lessons on its origins from the lesion itself. Nat. Rev. Gastroenterol. Hepatol. 12, 50–60 (2015).
Wang, X. et al. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell 145, 1023–1035 (2011).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 (2012).
Lee, Y. et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett's-like esophagus. Oncotarget 8, 203–214 (2017).
Spechler, S. J. et al. A summary of the 2016 James W. Freston Conference of the American Gastroenterological Association Intestinal Metaplasia in the Esophagus and Stomach: Origins, Differences, Similarities and Significance. Gastroenterology 153, e6–e13 (2017).
Yoshizawa, N. et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab. Invest. 87, 1265–1276 (2007).
Jensen, P. & Dymecki, S. M. Essentials of recombinase-based genetic fate mapping in mice. Methods Mol. Biol. 1092, 437–454 (2014).
Feil, R., Wagner, J., Metzger, D. & Chambon, P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757 (1997).
Grun, D. et al. De novo prediction of stem cell identity using single-cell transcriptome data. Cell Stem Cell 19, 266–277 (2016).
Goldenring, J. R. & Mills, J. C. Isthmus time is here: Runx1 identifies mucosal stem cells in the gastric corpus. Gastroenterology 152, 16–19 (2017).
Acknowledgements
J.B.S. holds a Postdoctoral Enrichment Program Award from the Burroughs Wellcome Fund and is also supported by the American Gastroenterology Association Gastric Cancer Foundation Research Scholar Award. J.C.M. is supported by National Institute of Diabetes and Digestive and Kidney Diseases awards DK094989, DK105129 and DK110406, by the Alvin J. Siteman Cancer Center–Barnes Jewish Hospital Foundation Cancer Frontier Fund, NIH National Cancer Institute P30 CA091842 and The Barnard Trust.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Superficial response
-
How the stomach (both corpus and antrum) repairs erosive injury (most commonly, owing to acid) on the epithelial surface.
- Surface epithelium
-
Mucus-secreting cells that line the surface of the stomach; also referred to as surface, foveolar or pit cells.
- Glandular response
-
How the stomach adapts to injury involving loss of acid-secreting parietal cells and digestive enzyme-secreting chief cells from gastric glands in the corpus.
- Gastric pits
-
The surface epithelium invaginates into gastric units that are funnel-shaped and dive downward towards the gastric muscular wall. The mouth-like opening of each gastric unit represents the gastric pit; the zone where the pit narrows into the gland harbours actively dividing stem cells and is called the isthmus.
- Gastric glands
-
We use here the human pathology definition of the gastric gland as being separate from the gastric pit. The glandular portion of a gastric unit is located at the base (that is, nearest the stomach muscular wall) and extends up to the isthmus. In the corpus, the gastric gland comprises parietal, chief, mucous neck and endocrine cells. In the antrum, the gastric gland contains mucous and endocrine cells.
- Achlorhydria
-
The absence of hydrochloric acid in gastric secretions.
- Ménétrier disease
-
A rare gastric condition characterized by hypertrophied gastric folds, hyperplasia of the surface epithelium and hypochlorhydria or achlorhydria.
- Oxyntic atrophy
-
A process characterized by the loss of acid-producing, or oxyntic, glands from the corpus.
- Zollinger–Ellison syndrome
-
A clinical syndrome defined by gastric acid hypersecretion owing to a gastrin-producing tumour (that is, gastrinoma).
- Hypochlorhydria
-
Decreased or low hydrochloric acid in gastric secretions.
- Transdifferentiation
-
The conversion of a cell type of one lineage to a cell type of a different lineage.
- Atrophic front
-
The stomach-adapted bacterium Helicobacter pylori is known to cause atrophy and metaplasia of the corpus in a subset of chronically infected patients; this atrophy spreads along a front from the antrum into the corpus along the lesser curvature.
- Cyclical hit model of tumorigenesis
-
A proposal that mutations can accumulate and be stored in differentiated cells. Following injury, differentiated cells can re-enter the cell cycle and proliferate. During their proliferative phase, mutations can be acquired. As cells re-differentiate, the acquired mutations are stored. These stored mutations may accumulate with little effect until the cells either undergo apoptosis or become trapped in a (proliferative) dysplastic state.
Rights and permissions
About this article
Cite this article
Sáenz, J., Mills, J. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol 15, 257–273 (2018). https://doi.org/10.1038/nrgastro.2018.5
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2018.5
This article is cited by
-
Single-cell transcriptomics uncovers EGFR signaling-mediated gastric progenitor cell differentiation in stomach homeostasis
Nature Communications (2023)
-
The composition and roles of gastric stem cells in epithelial homeostasis, regeneration, and tumorigenesis
Cellular Oncology (2023)
-
Applications of human organoids in the personalized treatment for digestive diseases
Signal Transduction and Targeted Therapy (2022)
-
Gastroduodenal Injury: Role of Protective Factors
Current Gastroenterology Reports (2019)
-
Helicobacter pylori infection and gastric cancer biology: tempering a double-edged sword
Cellular and Molecular Life Sciences (2019)