Abstract
Environmental enteric dysfunction (EED) is a disease of the small intestine affecting children and adults in low and middle income countries. Arising as a consequence of repeated infections, gut inflammation results in impaired intestinal absorptive and barrier function, leading to poor nutrient uptake and ultimately to stunting and other developmental limitations. Progress towards new biomarkers and interventions for EED is hampered by the practical and ethical difficulties of cross-validation with the gold standard of biopsy and histology. Optical biopsy techniques — which can provide minimally invasive or noninvasive alternatives to biopsy — could offer other routes to validation and could potentially be used as point-of-care tests among the general population. This Consensus Statement identifies and reviews the most promising candidate optical biopsy technologies for applications in EED, critically assesses them against criteria identified for successful deployment in developing world settings, and proposes further lines of enquiry. Importantly, many of the techniques discussed could also be adapted to monitor the impaired intestinal barrier in other settings such as IBD, autoimmune enteropathies, coeliac disease, graft-versus-host disease, small intestinal transplantation or critical care.
Similar content being viewed by others
Main
Environmental enteric dysfunction (EED) is a syndrome of the small intestine that is endemic in children and adults across the developing world1. Although it is by definition asymptomatic, EED in children is strongly associated with growth stunting and poor developmental outcomes1,2,3,4. The syndrome manifests as a global disturbance of gut function, leading to impaired intestinal barrier function and poor uptake of nutrients2,3,4. Environmental origins are suspected, but the aetiology is still not well understood and the most effective route to prevention and treatment remains unclear5,6.
The huge global burden of EED and our poor understanding of the disease motivate the discovery of new biomarkers for diagnosis and monitoring, and particularly for evaluating the efficacy of interventions. Validating these biomarkers, however, is challenging, as the gold standard of endoscopic biopsy and histology is too invasive to be used in large-scale research studies in children. A potential alternative route is via 'optical biopsy' techniques, which enable diagnostic information to be obtained less invasively. Here, rather than excising tissue for subsequent analysis in a laboratory, optical probes are used to make measurements on tissue in situ.
In this Consensus Statement, we summarize the current state of knowledge of EED and identify the characteristics that can be measurable by optical biopsy. We then review the most relevant optical biopsy techniques, including a description of the underlying technologies and their potential role in studying EED. We conclude with a set of recommendations for further research and development in this area, with a view to enabling clinical studies.
Methods
This Consensus Statement arose from a horizon scan project funded by the Bill and Melinda Gates Foundation to investigate potential applications of optical biopsy in tackling gut disease in developing countries. The horizon scan was undertaken at Imperial College London, UK, and most of the co-authors were invited to join the project advisory board on the basis of their extensive experience in relevant fields (T.T., W.A.F., T.J.M., P.D., S.L., A.M., R.R.-K., G.J.T. and P.K.). Other co-authors were invited to contribute as additional experts (L.S.C., C.L. and F.L.), based on the recommendations of the advisory board. Members of the board and experts met to discuss the horizon scan in London, UK, in November 2015 and San Francisco, USA, in February 2016. Literature searches were performed using Web of Science, as well as through the advice of the advisory board and experts. The results of the horizon scan are presented in this Consensus Statement, which entails a review of EED, a discussion of technologies deemed relevant to EED (based on literature searches and the opinions of the advisory board and experts), and a series of recommendations for the future application of the relevant technologies to the study and diagnosis of EED. In the Recommendations section, each technology has been assessed according to its suitability for application to EED and this assessment includes an evaluation of the cost, invasiveness and appropriateness for developing countries of each device and/or technique. This assessment was performed in a qualitative manner based on currently available information (for example, the present cost and size of the devices) and the opinions of the authors as to feasible future development. Technique suitability for use in developing countries was determined in a qualitative manner after consideration of the need for advanced infrastructure and high levels of training for either system use or data analysis. Probable deployment timescales have also been assigned to each technique based on the current states of development and the degree of validation work that will be required before application to EED. These timescales have been tentatively defined as short-term (suitable for deployment immediately or within ∼1 year), medium-term (suitable for deployment within ∼1–3 years) and long-term (suitable for deployment in 3 years or more). A technological readiness level (TRL) is also reported for each technology and this score is discussed further in the Recommendations section.
EED
Before the 1960s, understanding of small intestinal disorders such as coeliac disease had been hindered by the difficulty of obtaining biopsy samples. Newly developed techniques, such as the Crosby capsule, then began to reveal differences between the intestinal villous morphology of residents of developed and developing countries, particularly individuals in India and sub-Saharan Africa. These changes were to some extent reversible if an individual moved between regions7. This 'tropical enteropathy' was later understood to be more closely linked to economic conditions than geographical location, and was re-termed 'environmental enteropathy' or EED, with the latter term emphasizing that it is characterized by a global disturbance of gut function7.
EED is associated with a number of undesirable developmental outcomes, including poor response to vaccines and increased susceptibility to infection8, and most strongly with impaired growth velocity and stunting9,10. It is now thought that EED is an important component of the link between poor environmental conditions and reduced height, and a cause of the limited success of nutritional interventions in overcoming this growth deficit in many populations11. Although stunting in itself is not particularly harmful to the individual, it is a highly visible manifestation of the effect of EED, and correlates with a much wider range of poor socioeconomic outcomes, including poor performance in education, reduced income in adulthood and increased fertility1,11,12,13. Earlier diagnosis of EED, even in infants or very young children, could enable targeted interventions.
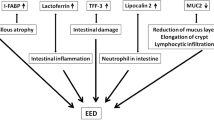
Diagnosis of EED. Definitive diagnosis of EED is currently by endoscopic biopsy and histology, and the histopathological features indicative of EED include: blunting of villi; increased crypt depth (although some cases show a decrease); reduced villus compartment surface area; increased cellular infiltrate, predominantly lymphocytes and plasma cells; epithelial monolayer fragility and breaks; tight junction and cell adhesion abnormalities; and increased epithelial cell shedding14. These features are shown in Fig. 1.
a | Healthy intestine. b | Changes to intestinal tissue associated with environmental enteric dysfunction.
Given the practical and ethical difficulties associated with the use of endoscopic biopsy in infants and young children, there has been a considerable drive towards identification of biomarkers of EED that can be monitored less invasively. The 2015 Technical Meeting on EED15,16 established several descriptive domains of EED (Box 1) in addition to the structural changes seen in histology, and any practical approach involving optical biopsy is likely to target one or more of these features.
Biomarkers for EED. The most commonly employed EED biomarker is the lactulose:mannitol (L:M) test, used to measure gut absorptive and barrier function17. Lactulose and mannitol are administered orally, and urine samples are subsequently collected for up to 6 h. Lactulose — a large disaccharide molecule — is not normally absorbed by the healthy small intestine. Mannitol, on the other hand, is a smaller monosaccharide that passes the healthy intestinal barrier and is found in urine17. An increased ratio of lactulose to mannitol in the urine, determined by liquid chromatography and/or mass spectrometry, therefore indicates one or both of reduced absorption or impaired intestinal barrier function. However, in practice the results depend strongly on the proficiency of those carrying out the test and the level of diligence observed in infant dietary fasting and regular urine collections17, and the test does not provide the same direct information as histology.
Many other biomarkers have been proposed as candidates to monitor or diagnose EED (comprehensive reviews are available elsewhere14,18) and new biomarkers are constantly being sought, but there is difficulty in obtaining a 'gold standard' measurement for comparison. Two large-scale trials are also currently investigating the effect of community-level water and sanitary health (WASH)19 improvements on factors including child-length for age, child development scores and stunting prevalence (the SHINE trial in Zimbabwe20 and the WASH Benefits Trial in Kenya and Bangladesh21). These studies will indicate the degree to which sanitary conditions drive EED and stunting, and hence the level of prevention that could be expected from widespread WASH improvements. Nevertheless, it is not possible to directly link sanitation to specific histological features of EED given the ethical and practical difficulties associated with performing longitudinal studies requiring repeated invasive procedures (under sedation) on asymptomatic infants and children in a developing world setting. Conversely, correlation with long-term developmental outcomes is inherently difficult, time-consuming and subject to confounding influences.
The potential benefit of optical biopsy therefore lies in providing comparable (or superior) information to histology in a less invasive manner. In the following section we highlight technologies with the potential to provide real-time diagnostic information in the small intestine. We then propose specific applications in the measurement of the domains of EED, with consideration of the practical issues related to deploying these techniques in the affected geographical regions. The principal focus is on techniques suitable for limited use within research studies aiming to identify noninvasive biomarkers or treatments, although we also suggest some potential point-of-care tests suitable for more widespread use.
Optical biopsy for the small bowel
Numerous optical biopsy techniques have been reported in the literature, but relatively few have been employed in the small intestine, and hardly any specifically for EED. The optical imaging and spectroscopic techniques included below are therefore only those which have demonstrated potential for use in this setting, either because they can perform measurements relevant to the diagnosis of EED or because there is a history of clinical use related to other small bowel conditions, such as coeliac disease. For each technology we summarize the underlying principles, assess the technological readiness for clinical applications, and identify potential applications in EED.
Endoscopy and capsule endoscopy. Endoscopy is widely used to remotely visualize regions of the gastrointestinal tract, including the small intestine. Most modern endoscopes use incoherent fibre-optic bundles or distal light emitting diodes (LEDs) to illuminate the tissue and miniature cameras to capture live video. Alternatively, wireless capsule endoscopy22, which provides live imaging via an untethered ingested capsule transiting the gastrointestinal tract, has been available since the early 2000s. This approach is considerably less invasive than standard endoscopy, but offers poorer imaging resolution and is non-steerable.
Although biopsies are required for definitive histopathological diagnoses, endoscopy can resolve mucosal structures such as duodenal villi, suggesting that it could identify features of EED. Indeed, endoscopic imaging has been applied to numerous studies of coeliac disease, which exhibits similar but more extreme changes in intestinal structure to EED, including scalloped duodenal folds, grooves, fissurations and loss of the finger-shaped villi that cover the healthy mucosal surface23.
The contrast of the villous structure can be enhanced through techniques including water immersion24, high-definition (HD) endoscopy25, chromoendoscopy26, narrow band imaging (NBI)27, and other forms of optical or digital chromoendoscopy such as optimal band imaging (OBI)28, providing sensitivities and specificities of up to 100% for villous atrophy25. Figure 2a–d shows the contrast improvement offered by optical chromoendoscopy, revealing clear differences between healthy and unhealthy villous mucosa. Furthermore, HD endoscopy has been applied to preliminary studies of EED in adults (Fig. 2e,f), in which it has been shown capable of identifying changes in the villous architecture that are associated with EED.
a | Image illustrating the improved villous contrast provided by optimal band imaging (OBI) and high definition (HD) endoscopy. b | An equivalent image utilizing HD endoscopy alone. c | Narrow band imaging (NBI) of regions of tissue exhibiting healthy villi. d | NBI images showing regions of tissue with absent villi. e | HD endoscopy image recorded in an adult with EED exhibiting partial blunting of villi. f | HD endoscopy image in an adult with EED showing subtotal villous atrophy. Parts a,b reprinted from Gastrointestinal Endoscopy, 68, Cammarota, G. et al., Optimal band imaging system: a new tool for enhancing the duodenal villous pattern in coeliac disease, 352–357, Copyright 2008, with permission from Elsevier. Parts c,d © Georg Thieme Verlag KG, reprinted from Ref. 26. Parts e, f provided by P.K.
Although endoscopic examinations can be performed safely in infants29 and even neonates30, this procedure is considerably more challenging — and has higher associated risks — than when undertaken in adults or older children31, even when using the thinnest (<6 mm diameter) paediatric endoscopes. For this reason, endoscopy in children is almost always performed using either deep sedation or anaesthesia (which have their own associated risks32). It would therefore be ethically difficult to undertake such an invasive procedure for research purposes in asymptomatic infants who would derive no immediate benefit. This issue limits the role of endoscopy to studies in adults or in very small groups of infants (for example, in children who are undergoing endoscopy and/or sedation for other reasons).
Capsule endoscopy has previously been deployed in children to investigate gastrointestinal bleeding, Crohn's disease, coeliac disease and small bowel polyps33. The technique is approved by the FDA for use in children ≥2 years of age34 and safe use has been demonstrated in infants as young as 10 months35. Capsules are inherently less invasive than standard endoscopes as they move through the body via natural motion induced by peristalsis rather than being manually manoeuvred by the clinician. Thus, they are less likely to cause damage to the intestinal epithelium during use22. Importantly, endoscopic placement of the capsule can enable use of capsule endoscopy in children who are unable to swallow the device36, although this method would obviate the benefit of the non-endoscopic approach to some degree.
A review by Ianiro et al.25 reported the sensitivity and specificity of capsule endoscopy for the diagnosis of villous damage in patients with coeliac disease as 70.0–95.2% and 63.6–100% respectively, lower values than typical for HD or NBI endoscopy, but nevertheless representing good diagnostic accuracy. Villous atrophy was indirectly measured via assessment of image mosaicism25. Although this approach could be considered a limitation, it has the benefit of permitting automated image analysis37 for use in low-resource settings, and these quantitative results can be matched to the Marsh criteria (a classification based on the presence of intraepithelial lymphocytes, crypt hyperplasia and villous atrophy38) that are used in conventional histological diagnosis of coeliac disease37. However, the risk of capsule retention is ∼1%33,39, a potential complication that might need to be mitigated with tethered capsules or ingestible radio frequency identification tags for device localization40. Additionally, preliminary studies in adults would be required to compare capsule measurements to standard endoscopic assessments of villous atrophy.
Fluorescence endomicroscopy. Fluorescence endomicroscopy provides high resolution images of tissue in vivo via flexible probes41,42 and is one of the most widely used and clinically validated optical biopsy techniques (Fig. 3). Unlike standard or even HD endoscopy, endomicroscopy enables direct identification of morphological changes in the tissue microstructure following application of topical or intravenous fluorescent stains. Most clinical studies use optically-sectioning 'confocal' endomicroscopy, whereby a focused laser spot is scanned across the tissue and an image is built up point-by-point. Focusing the collected light through a pinhole before the detector results in rejection of out-of-focus light and a high contrast image41.
a | Distal scanning endomicroscopy. b | Proximal scanning endomicroscopy. The proximal scanning approach results in a smaller probe tip that can be used with paediatric endoscopes. c | Cellvizio image of the terminal ileum acquired using intravenous fluorescein staining. d | ISC-1000 image of the duodenal mucosa acquired using intravenous fluorescein staining. e | ISC-1000 image of the small intestinal mucosa acquired using intravenous fluorescein staining in an adult with EED, showing leakage of fluorescein through the epithelium (arrow). f | A cell shedding event (arrow) in the same patient, also visualized using the ISC-1000 system. Part c reprinted from Gastrointestinal Endoscopy, 77, Turcotte, J.-F. et al., Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos), 624–630, Copyright 2013, with permission from Elsevier. Part d reprinted from Baishideng Publishing Group Inc, Venkatesh, K. et al. World J. Gastroenterol. 15, 2214–2219 (2009). Parts e,f provided by P.K.
Optical fibres are used for light delivery and collection, and laser scanning is achieved using either a distal43,44 or proximal45 mechanism. Miniaturized distal scanning mechanisms involve rapidly scanning the tip of a single fibre in two dimensions, using miniaturised magnetic or piezo actuators (Fig. 3a). By contrast, in proximal scanning, conventional 2D scanning mechanisms, such as a pair of galvanometer scanners, are used externally (Fig. 3b). The scanning pattern is then relayed to the tissue via a flexible bundle containing up to 30,000 fibres, which acts as an image conduit. Distal scanning can provide high resolution images, generally at low frame rates, whereas proximal scanning (using the fibre imaging bundle to relay the scanning pattern from an external system) enables for sub-mm diameter probes at the expense of resolution. Importantly, these sub-mm proximal scanning probes are compatible with paediatric endoscopes, as they can be inserted through the 2 mm diameter working channel. The distal scanning approach is used by the ISC-1000 (Pentax, Japan), a 5 mm diameter endomicroscope integrated into a Pentax endoscope that offers an adjustable working distance for 3D imaging, although this device is no longer commercially available. The proximal scanning method is used in the Cellvizio system (Mauna Kea Technologies, France) and several variations of this system are now available46,47,48, with the thinnest probe offered by Mauna Kea Technologies having an outer diameter of 0.85 mm (Ref. 49). Simple non-scanning endomicroscopes, using a fibre-bundle to flood illuminate the sample and then collect images, have also been applied to the gastrointestinal tract50,51 but not commercialized.
Studies in the small intestine, both for coeliac disease and EED itself, have shown that both the ISC-1000 (Ref. 52) and the Cellvizio53,54 systems can resolve villous structures, the capillary network and both goblet and columnar epithelial cells (Fig. 3d,e) when using intravenous fluorescein as a contrast agent. Other studies have shown that endomicroscopy can diagnose coeliac disease55,56,57,58 based on recognition of features relevant to EED, such as villous atrophy, crypt hyperplasia and lymphocyte infiltration. In particular, Venkatesh et al.58 reported successful diagnosis of coeliac disease (100% sensitivity, 80% specificity) in children as young as 1.8 years of age. Semi-automated methods for the detection and segmentation of villi56 and for the measurement of goblet cell density57 have also been presented.
Fluorescence endomicroscopy also enables observation of gaps or breaks in the intestinal epithelial lining59,60,61. For patients with IBS60,61 the leakage of intravenous fluorescein into the lumen has been visualized in real time61, indicating that endomicroscopy can be used to monitor dynamic, cellular level changes in gut function and permeability. In a study of EED in 41 Zambian adults using the Pentax ISC-1000 endomicroscope4, only 8 (20%) exhibited no luminal leakage of fluorescein and only 2 (5%) showed no fluorescein plumes. By comparison, in a healthy population, 80% showed no fluorescein leakage or plumes (P < 0.0001)62. Endomicroscopy was also used to quantify single and multiple cell defects, cell shedding events, apoptotic cells and epithelial breakdown lesions in patients with severe and minimal EED, and these features were correlated to the expression of 23 genes that were found to be differentially expressed in intestinal epithelium4.
Fluorescence endomicroscopy therefore has the potential to provide information relevant to EED, including structural changes in the small intestine and measurements of gut permeability. Although advanced systems with 3D imaging capabilities could be particularly useful in early studies, such experiments might be restricted by the lack of a commercially available instrument. Thus, smaller diameter fibre-bundle-based systems will probably be more convenient and will certainly be most suitable for use in children (due to their small size and compatibility with paediatric endoscopes). However, endomicroscopy requires use of an endoscope and so is likely to be limited to research studies in adults or small groups of children (possibly those undergoing endoscopy and/or sedation for alternative reasons). Less invasive imaging could be possible using a tethered fluorescence endomicroscopy capsule (similar to the reflectance confocal microscopy capsule63), but a practical system is yet to be demonstrated.
Endoscopic optical coherence tomography. Endoscopic optical coherence tomography (OCT)64 provides high resolution, high speed, cross-sectional images to a tissue depth of 1–2 mm. It is predominantly a structural imaging tool, able to visualize the morphology of both villi and crypts in the small intestine65. The advantages over endomicroscopy are its high speed (allowing rapid interrogation of large areas of tissue) and the ability to obtain cross-sectional images, although it is unable to visualize fluorescent dyes.
Detailed descriptions of the principles of OCT are available elsewhere (for example Ref. 66), but, briefly, it involves illuminating tissue with a low coherence light source and using interferometry to select (coherence gate) light returning from a chosen depth layer. By scanning the position of the coherence gate it is then possible to generate a depth scan along the z-axis into tissue (also known as an A-scan). Axial resolutions as low as approximately 1 μm are achievable, although 10–20 μm is more common for endoscopic systems. Volumetric imaging is enabled by lateral scanning in two dimensions (with lateral resolutions typically on the order of 10–20 μm)66. Modern systems use Fourier domain techniques to achieve A-scans at hundreds of kHz or even MHz (Ref. 67) rates by acquiring data from all depths simultaneously.
OCT is inherently fibre compatible and a wide range of endoscopic systems have been developed. The main challenge is the fabrication of a high speed lateral scanning mechanism at the distal tip. A number of forward viewing probes have been reported (for example Ref. 68) using similar approaches to distal scanning endomicroscopes, and although these have been used in the gastrointestinal tract (for example Ref. 69), side viewing devices are more suited to imaging large areas of lumina. Side viewing probes are readily fabricated by incorporating a right angled prism and one or more micro-lenses at the distal tip of a fibre. Proximally driven rotation of the fibre probe, or rotation of the prism using a micro-motor, generates circular cross-sectional images, while pull-back of the probe enables collection of volumetric datasets70 over large regions of tissue. Probes with diameters <1 mm are achievable — allowing deployment through either standard or paediatric endoscopes and catheters — and have been applied extensively to vascular imaging (for example Ref. 71) and within the gastrointestinal tract (for example Ref. 72).
Tethered capsule OCT (also referred to as tethered capsule endomicroscopy, TCE) was reported by Gora et al.73 in 2013. The capsule (which measures 24.8 mm by 12.8 mm) is swallowed by the patient, rather than being introduced by endoscope, and provides 3D imaging via a pull-back manoeuvre (Fig. 4). This technology is considerably less invasive than endoscopy, as the capsule relies on natural peristalsis to pass through the gastrointestinal tract rather than being externally manoeuvred, and can be undertaken in unsedated (adult) patients73. TCE has been applied to the assessment of Barrett oesophagus, with volumetric images of the entire oesophagus collected in as little as 60 s (Ref. 74). There have also been initial reports of extending TCE to rapid, minimally invasive imaging of the small intestine75.
a | A tethered capsule optical coherence tomography (OCT) system. PP, polypropylene ball handle. b | 3D view of the entire oesophagus in a patient with Barrett oesophagus, obtained via 3D rendering of data collected as the OCT capsule is withdrawn from the stomach. Distinct features are revealed by OCT including normal gastric mucosa (left cross-sectional image), high-grade dysplasia (HGD) and intramucosal carcinoma (IMC) (centre), and normal squamous mucosa (right). c | Probe-based OCT image collected in the duodenum (left) and corresponding histological image (right) indicating visualisation of the villous structure in healthy tissue. Finger-like villi are clearly resolved (arrows). d | Probe-based OCT image (left) showing areas of villous atrophy (arrows) exhibiting a flattened mucosal surface, and the corresponding histological image (right). Parts a,b adapted with permission from Macmillan Publishers Ltd, part of Springer Nature © Gora, M. J. et al., Nat. Med. 19, 238–240 (2013). Parts c,d reprinted from Digestive and Liver Disease, 41, Masci, E. et al., Optical coherence tomography in pediatric patients: A feasible technique for diagnosing coeliac disease in children with villous atrophy, 639–643, Copyright 2009, with permission from Elsevier.
Although there have been few in vivo OCT investigations of the small intestine, a 2005 study65 using an endoscopic probe-based system demonstrated clear visualization of villi. In addition, a subsequent attempt to diagnose coeliac disease using this technique by assessing villous atrophy76 reported a sensitivity and specificity of 100% and 82%, respectively, in a group of 67 children aged 1–14 years. Images from this study are shown in Fig. 4c,d.
OCT therefore seems to offer sufficient resolution for the assessment of changes in the tissue microstructure that are relevant to EED. Moreover, tethered capsule OCT systems provide minimally invasive, high speed imaging and could be applied to large cohorts of adults to obtain an unprecedented level of information from large sections of the small intestine. This approach could also be extended to children and infants, with the risk of capsule retention mitigated by the tether. However, endoscopic introducers are probably necessary in young children to aid swallowing of the capsule, which might be challenging for large-scale implementation. Nonetheless, the deployment of TCE in small numbers of children could be feasible and could help to provide vital information on the changes that occur in intestinal tissue microstructure during the early stages of development. Interestingly, if smaller capsules are desirable for paediatric use then their development should be feasible, as the combined diameter of all optical and mechanical components contained within the capsule is already considerably smaller than that of the capsule itself (Fig. 4a). For example, 1 mm external diameters have been achieved in the side-viewing vascular or endoscopic OCT probes discussed earlier in this section, which use near-identical technology to that used in capsule OCT.
Fluorescence and reflectance spectroscopy. Optical spectroscopy entails illuminating tissue with light of a known wavelength and recording the intensity of the returning signal, such as fluorescence or scattered light, as a function of wavelength (these principles are described in detail elsewhere77). Although 2D spectral imaging is possible, spectroscopic clinical studies often involve single point measurements only, sacrificing spatial resolution to achieve acceptable acquisition times and to minimise probe complexity.
Although fluorescence and reflectance spectroscopy (along with various further spectroscopic techniques) have been applied to disease studies in the gastrointestinal tract using fibre-based endoscopic probes, this is unlikely to represent the most promising application for EED. Endoscopic spectroscopy could certainly be used to monitor the metabolic status of the gut via fluorescence measurements (for example, of the relative fluorescence intensities or the fluorescence lifetimes of the enzymatic cofactors NADH and FAD)78,79,80. In addition, a variety of reflectance-based modalities (including diffuse reflectance spectroscopy81,82 and elastic scattering spectroscopy83) could enable assessment of the tissue oxygen saturation and the haemoglobin concentration.
It is possible that both metabolic measurements (for example of the tissue redox ratio79,80) and quantification of the haemoglobin concentration and/or tissue oxygen saturation could be relevant to EED, but this applicability is yet to be confirmed experimentally. Another potential use of reflectance spectroscopy in EED could lie in determining some of the morphological changes relevant to the disease, such as the degree of cellular infiltration or the prevalence of breaks in the epithelial monolayer. Light scattering spectroscopy84,85,86 and low-coherence enhanced backscattering spectroscopy87 have both been used to assess aspects of cellular and tissue morphology (in vivo in the human oesophagus, colon and duodenum) and, as such, it might be possible to extend these techniques to EED. Fibre-optic fluorescence spectroscopy could also be used to monitor the leakage of intravenous fluorescent dye into the gut lumen in a similar manner to that described for fluorescence endomicroscopy. In all cases, however, these spectroscopic techniques will not provide spatial resolution and, for that reason, would be inferior to their competitors. Furthermore, although the reduced complexity of single point spectroscopy probes means that their outer diameters can often be smaller than those of fluorescence endomicroscopes, they still require an endoscope for deployment and, hence, can be considered equally invasive. In addition, endomicroscopy and OCT systems are (in some cases) commercially available and currently have a higher level of clinical penetration than endoscopic spectroscopy devices, implying that their deployment for research studies will be easier. Thus, it is likely that endomicroscopy and OCT will prove more useful for investigating changes in the morphology and the permeability of intestinal tissue in EED than in vivo endoscopic spectroscopy.
An alternative, more promising application of fluorescence spectroscopy in EED could be to monitor gut permeability in a minimally invasive or noninvasive manner from outside of the body, without the use of an endoscope. A number of studies have reported the use of microscopy or spectroscopy combined with fluorescent or reflective microparticles to monitor intestinal closure, transport and translocation in animals88,89,90,91,92,93. In general, the protocols used in these investigations first involved feeding animals a dose of a contrast agent such as fluorescein isothiocyanate (FITC)-labelled dextran88,89, fluorescently tagged polystyrene microspheres90,91, colloidal gold nanoparticles92 or iron oxide nanoparticles93. The uptake of contrast agent from the intestine into the blood, or into specific cells or tissues, was then assessed using spectroscopy or microscopy. Importantly, whereas many of these studies relied upon microscopic examination of ex vivo tissue samples to determine the degree of uptake, the work by Weström et al.88 and Ekström et al.89 demonstrated direct monitoring of the blood serum concentration of fluorescein (in ex vivo blood samples) using fluorescence spectroscopy.
Translating this approach into in vivo human use could be possible using a number of methodologies (Fig. 5), the most promising of which would involve the use of fluorescence spectroscopy to make noninvasive measurements of gut leakiness. In this scenario, the patient would receive an oral dose of contrast agent and the concentration of the agent in the blood would be measured some time later using a transcutaneous spectrometer. Such a device could entail a fingerclip system (similar to many commercially available pulse oximeters) or a skin patch. Both could be fabricated at low cost and would be almost entirely noninvasive. In addition, measurements of fluorescent dyes conjugated to particles of varying sizes would permit semi-automatic quantification of gut permeability. Validation for application to EED could be achieved by combining spectral measurements with L:M tests and/or morphological imaging. Importantly, after successful validation, transcutaneous fluorescence spectroscopy will be suitable for noninvasive monitoring of gut permeability in large cohorts of infants for studies such as the assessment of nutritional or WASH interventions.
FITC, fluorescein isothiocyanate.
Photoacoustic imaging. Photoacoustic imaging (PAI) — which relies upon the photo-acoustic effect, whereby a material absorbing light emits acoustic waves94 — involves exciting tissue with nonionizing laser pulses. When absorbed by chromophores in the tissue, this excitation generates heat, expansion of material and subsequent emission of ultrasound that can be detected with an ultrasonic transducer95,96. Both endogenous chromophores, such as oxyhaemoglobin and deoxyhaemoglobin, and exogenous contrast agents can be used, enabling PAI to provide multifaceted data containing both structural and functional information.
As this technology is based upon the detection of ultrasonic pressure waves, PAI affords considerably higher tissue penetration depths than other optical imaging modalities. There is a trade-off between penetration depth and resolution, but sub-mm resolution is achievable at depths of up to a few cm (Ref. 96). Penetration of a few cm would be sufficient to image the intestine of an infant from outside of the body, and so PAI might provide another route towards noninvasive investigation of EED, although this application remains highly speculative.
Although numerous chromophores (endogenous or exogenous) can be imaged using PAI, a probable application to EED is in imaging of gut permeability. This procedure would require the use of exogenous contrast agents and would entail protocols similar to those described earlier for fluorescence spectroscopy. One approach would involve imaging the leakage of an intravenously delivered photoacoustic contrast agent from the blood stream into the gut using endoscopic probes. Several endoscopic PAI probes have been reported97 and a number have been applied to gastrointestinal studies, both in live animals98 and in ex vivo samples of human tissue99, suggesting that this technique would be entirely feasible. Alternatively, noninvasive PAI could be achieved by using an orally ingested contrast agent along with an external imaging probe (likely to consist of a pulsed excitation laser combined with a commercially available ultrasound transducer similar to the devices reported in Ref. 100 and Ref. 101). Unlike transcutaneous fluorescence spectroscopy, PAI could permit spatial mapping of the leakiness of the intestine, hence offering a more detailed assessment of the permeability of the gut. Although PAI using an external imaging probe could potentially be applicable to large scale studies in children (due to its noninvasive nature), it is an immature technology and substantial developmental work will be necessary, for example in combining PAI with other measurement protocols for validation. Nonetheless, due to the potential utility for EED, this development and validation is likely to be worthwhile.
Recommendations
Although each of the techniques described above has potential applications in EED, they differ not only in terms of their format, the information they provide, and the type and scale of study they will be most appropriate for, but also in their technological maturity. The TRLs of each technology are listed in Table 1, along with additional information including the expected development timescales. The TRL scale (adapted from a scale originally developed by NASA) ranges from 1 to 9, with '1' indicating an initial concept and '9' representing a mature, commercialized device102. Other factors relevant to the adoption of these techniques for EED are also listed, including cost, invasiveness and suitability for developing world use. These additional factors are particularly important for large scale studies (given issues that can be expected at scale, such as the need for widespread deployment and the analysis of large datasets), although less-so for early aetiological investigations in small groups of patients. The likelihood of a technology finding other uses in more developed healthcare systems is also a consideration, as this affects the chances of subsequent commercial investment, as well as providing additional routes for testing and validation.
As can be seen from the TRLs in Table 1, the timescale over which techniques could be applied to EED varies markedly. HD and NBI endoscopy and endomicroscopy both have high TRLs, being fully commercialized and widely deployed, and could be adopted for use in EED immediately. Both have the potential to provide vital new information on the structural changes that occur in the gut as a result of EED, and endomicroscopy has already been applied to the study of both gut structure and permeability in adults with EED4. Endoscopy and endomicroscopy could be applied in the short term to small-scale aetiological investigations, most practicably in cases where children are already undergoing sedation for other procedures (given the invasiveness of the proposed measurements).
Capsule endoscopy could also be used immediately, although some validation (for example, comparison to endoscopy or endomicroscopy) will be required in order to confirm the sensitivity and specificity of the technique in detecting villous atrophy. Subsequently, capsule endoscopy could be applied to studies in large cohorts as the procedure is considerably less invasive than standard endoscopy. Detection of villous atrophy can be achieved using automated image processing algorithms, removing the requirement for manual analysis by a specially trained pathologist. Although it is only approved for children older than 2 years of age, endoscopic introduction would enable capsule imaging in younger children and the development of smaller devices could allow this restriction to be challenged.
Transcutaneous fluorescence spectroscopy and tethered capsule OCT are both suitable for medium-term use (within ∼1–3 years). Tethered capsule OCT has already been shown to enable imaging of the entire oesophageal mucosa in a matter of minutes using a protocol that is considerably less invasive than endoscopy. Moreover, OCT is capable of resolving intestinal villi, making it a very promising tool for the investigation of EED due to its capability to evaluate many villi in one procedure. Further development will be required to enable intestinal imaging but this should not be a limiting factor. As with capsule endoscopy, use of OCT in children is feasible but will be limited to those aged 2 years and above. Again, this constraint can be mitigated by further miniaturization and the incorporation of endoscopic introducers into experimental protocols.
Fluorescence spectroscopy is also a promising technology due to its potential to be applied almost noninvasively. Fingerclip-style spectrometers could be used to monitor the leakage of orally administered contrast agents from the gut to the blood stream, providing functional information pertinent to EED. This approach will require validation, either using some of the high-TRL imaging instruments discussed earlier or L:M tests. Once this validation is complete, fluorescence spectroscopy will be applicable to large-scale studies in remote locations due to its practically noninvasive methodology, low cost, simplicity of data analysis and ease of deployment. Interestingly, there might be scope to extend this approach for use with multiple molecular probes to evaluate further aspects of intestinal pathophysiology.
Finally, PAI could in the future also offer a noninvasive experimental protocol for the assessment of gut permeability (similar to that described for fluorescence spectroscopy), with an external PAI probe used to image the small intestine from outside of the body. An advantage over spectroscopy could be the ability to obtain low-resolution spatial maps of gut permeability, although more advanced data analysis will be required (possibly necessitating a pathologist). Substantial research and development work is needed, both in device design and validation, meaning that PAI would only be deployable in the longer term (>3 years).
The list of optical biopsy techniques presented herein is not exhaustive and other approaches might be useful in the study of EED. One example is Raman spectroscopy, which has been used for the detection and identification of bacteria in previous investigations103,104 and could prove useful in establishing the make-up of the intestinal microbiota (for example, via measurements of tissue or faecal samples, or through in vivo endoscopic studies). Endocytoscopy, a form of white light endomicroscopy, can also visualize normal villi105, but cannot detect the functional changes visualized by fluorescence endomicroscopy. There is also a range of both optical and nonoptical (typically electrochemical) sensing techniques that enable simultaneous and selective measurements of metabolite (for example, lactate, glucose), electrolyte (for example, pH, sodium, potassium) or other biomarker concentrations in a variety of samples (such as blood, tissue, urine, saliva and sweat, for example in Ref. 106). These approaches could have uses, for example, in the detection of cytokines or other markers of inflammation that are relevant to EED. Indeed, some of these approaches (in particular electrochemical sensing and Raman spectroscopy) might complement the optical biopsy techniques discussed in this article, as they have the potential to provide measurements that are not possible with the methods highlighted here. For example, investigating the intestinal microbiome using Raman spectroscopy and assessing gene expression through electrochemical detection of specific biomarkers could both hold substantial merit in the study of EED. As such, all of the above techniques could be worthy of further investigation.
Conclusions
EED is a widespread condition in developing countries, in some cases affecting almost entire regional populations, and has severe negative impacts on the physical and cognitive development of children. Given that EED remains poorly understood, it is vital that new technologies and approaches are investigated and implemented to improve understanding of the disease and to help guide the development of new therapies. We have identified and assessed optical biopsy techniques with potential to assist in this area. Although many optical methodologies could potentially be applied, we have highlighted endoscopy (including HD, NBI and capsule endoscopy), fluorescence endomicroscopy, OCT, fluorescence spectroscopy and PAI as the most promising. These will provide data not only on structural changes in the intestine, but also on the degree of intestinal permeability. We have identified how each approach could be advanced towards this goal, and we expect each technique to offer important and unique information. The further investigation and development of all of these approaches could result in a pipeline of studies offering rich and varied information on this poorly understood disease.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ali, A., Iqbal, N. T. & Sadiq, K. Environmental enteropathy. Curr. Opin. Gastroenterol. 32, 12–17 (2016).
Prendergast, A. J. & Kelly, P. Interactions between intestinal pathogens, enteropathy and malnutrition in developing countries. Curr. Opin. Infect. Dis. 29, 229–236 (2016).
Prendergast, A. J. & Humphrey, J. H. The stunting syndrome in developing countries. Paediatr. Int. Child Health 34, 250–265 (2014).
Kelly, P. et al. Endomicroscopic and transcriptomic analysis of impaired barrier function and malabsorption in environmental enteropathy. PLoS Negl. Trop. Dis. 10, e0004600 (2016).
Trehan, I., Kelly, P., Shaikh, N. & Manary, M. J. New insights into environmental enteric dysfunction. Arch. Dis. Child. 101, 741–744 (2016).
Petri, W. A. Jr, Naylor, C. & Haque, R. Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Med. 12, 187 (2014).
Keusch, G. T. et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin. Infect. Dis. 59, S207–S212 (2014).
Acosta, A. M. et al. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin. Infect. Dis. 59, S193–S206 (2014).
Guerrant, R. L., DeBoer, M. D., Moore, S. R., Scharf, R. J. & Lima, A. A. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol. 10, 220–229 (2013).
Humphrey, J. H. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374, 1032–1035 (2009).
Dewey, K. G. & Adu-Afarwuah, S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern. Child Nutr. 4, 24–85 (2008).
Crane, R. J., Jones, K. D. & Berkley, J. A. Environmental enteric dysfunction: an overview. Food Nutr. Bull. 36, S76–S87 (2015).
Grantham-McGregor, S. et al. Child development in developing countries 1 — developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70 (2007).
Watanabe, K. & Petri, W. A. Jr Environmental enteropathy: elusive but significant subclinical abnormalities in developing countries. EBioMedicine 10, 25–32 (2016).
Nutritional and Health-Related Environmental Studies Section, Division of Human Health, International Atomic Energy Agency. Technical meeting on environmental enteric dysfunction, the microbiome and undernutrition (IAEA, 2015).
Owino, V. et al. Environmental Enteric Dysfunction and Growth Failure/Stunting in Global Child Health. Pediatrics 138, e20160641 (2016).
Denno, D. M. et al. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin. Infect. Dis. 59, S213–S219 (2014).
Syed, S., Ali, A. & Duggan, C. Environmental enteric dysfunction in children. J. Pediatr. Gastroenterol. Nutr. 63, 6–14 (2016).
Mbuya, M. N. N. & Humphrey, J. H. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries. Matern. Child Nutr. 12 (Suppl. 1), 106–120 (2016).
Prendergast, A. J. et al. Assessment of environmental enteric dysfunction in the SHINE trial: methods and challenges. Clin. Infect. Dis. 61, S726–S732 (2015).
Arnold, B. F. et al. Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH benefits study design and rationale. BMJ Open 3, e003476 (2013).
Iddan, G., Meron, G., Glukhovsky, A. & Swain, P. Wireless capsule endoscopy. Nature 405, 417 (2000).
Cammarota, G., Fedeli, P. & Gasbarrini, A. Emerging technologies in upper gastrointestinal endoscopy and celiac disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 6, 47–56 (2009).
Cammarota, G. et al. Reliability of the “immersion technique” during routine upper endoscopy for detection of abnormalities of duodenal villi in patients with dyspepsia. Gastrointest. Endosc. 60, 223–228 (2004).
Ianiro, G., Gasbarrini, A. & Cammarota, G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J. Gastroenterol. 19, 8562–8570 (2013).
Siegel, L. M. et al. Combined magnification endoscopy with chromoendoscopy in the evaluation of patients with suspected malabsorption. Gastrointest. Endosc. 46, 226–230 (1997).
Singh, R. et al. Narrow-band imaging in the evaluation of villous morphology: a feasibility study assessing a simplified classification and observer agreement. Endoscopy 42, 889–894 (2010).
Cammarota, G. et al. Optimal band imaging system: a new tool for enhancing the duodenal villous pattern in celiac disease. Gastrointest. Endosc. 68, 352–357 (2008).
Barth, B. A. et al. Equipment for pediatric endoscopy. Gastrointest. Endosc. 76, 8–17 (2012).
Volonaki, E. et al. Gastrointestinal endoscopy and mucosal biopsy in the first year of life: indications and outcome. J. Pediatr. Gastroenterol. Nutr. 55, 62–65 (2012).
Lightdale, J. R. et al. Modifications in endoscopic practice for pediatric patients. Gastrointest. Endosc. 79, 699–710 (2014).
American Academy of Pediatrics, American Academy of Pediatric Dentistry, Coté, C. J., Wilson, S. & Work Group on Sedation Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatr. Anaesth. 118, 2587–2602 (2006).
Cohen, S. A. et al. Pediatric capsule endoscopy: review of the small bowel and patency capsules. J. Pediatr. Gastroenterol. Nutr. 54, 409–413 (2012).
Thomson, M. et al. Wireless capsule endoscopy in children: a study to assess diagnostic yield in small bowel disease in paediatric patients. J. Pediatr. Gastroenterol. Nutr. 44, 192–197 (2007).
Oikawa-Kawamoto, M. et al. Safety and utility of capsule endoscopy for infants and young children. World J. Gastroenterol. 19, 8342–8348 (2013).
Barth, B. A., Donovan, K. & Fox, V. L. Endoscopic placement of the capsule endoscope in children. Gastrointest. Endosc. 60, 818–821 (2004).
Ciaccio, E. J. et al. Quantitative assessment of endoscopic images for degree of villous atrophy in celiac disease. Dig. Dis. Sci. 56, 805–811 (2011).
Marsh, M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 102, 330–354 (1992).
Liao, Z., Gao, R., Xu, C. & Li, Z. S. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest. Endosc. 71, 280–286 (2010).
Postgate, A. J., Burling, D., Gupta, A., Fitzpatrick, A. & Fraser, C. Safety, reliability and limitations of the given patency capsule in patients at risk of capsule retention: a 3-year technical review. Dig. Dis. Sci. 53, 2732–2738 (2008).
Jabbour, J. M., Saldua, M. A., Bixler, J. N. & Maitland, K. C. Confocal endomicroscopy: instrumentation and medical applications. Ann. Biomed. Eng. 40, 378–397 (2011).
Liu, J. T. C., Loewke, N. O., Mandella, M. J., Levenson, R. M., Crawford, J. M. & Contag, C. H. Point-of-care pathology with miniature microscopes. Anal. Cell. Pathol. (Amst.) 34, 81–98 (2011).
Dickensheets, D. L. & Kino, G. S. Scanned optical fiber confocal microscope. Proc. SPIE http://dx.doi.org/10.1117/12.172104 (1994).
Dickensheets, D. L. & Kino, G. S. Micromachined scanning confocal optical microscope. Opt. Lett. 21, 764–766 (1996).
Gmitro, A. F. & Aziz, D. Confocal microscopy through a fiber-optic imaging bundle. Opt. Lett. 18, 565–567 (1993).
Laemmel, E. et al. Fibered confocal fluorescence microscopy (Cell-viZio™) facilitates extended imaging in the field of microcirculation. J. Vasc. Res. 41, 400–411 (2004).
Thiberville, L. & Salaün, M. Bronchoscopic advances: on the way to the cells. Respiration 79, 441–449 (2010).
Vercauteren, T. et al. Multicolor probe-based confocal laser endomicroscopy: a new world for in vivo and real-time cellular imaging. Proc. SPIE http://dx.doi.org/10.1117/12.2002490 (2013).
Konda, V. J. A. et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy 45, 1006–1013 (2013).
Pierce, M. C., Vila, P. M., Polydorides, A. D., Richards-kortum, R. & Anandasabapathy, S. Low-cost endomicroscopy in the esophagus and colon. Am. J. Gastroenterol. 2011, 1722–1724 (2012).
Vila, P. et al. Accuracy and interrater reliability for the diagnosis of Barrett's neoplasia among users of a novel, portable high-resolution microendoscope. Dis. Esophagus 27, 55–62 (2014).
Polglase, A. L., Mclaren, W. J. & Skinner, S. A. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest. Endosc. 62, 686–695 (2005).
Wallace, M. et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 43, 882–891 (2011).
Nonaka, K. et al. Development of a new classification for in vivo diagnosis of duodenal epithelial tumors with confocal laser endomicroscopy: a pilot study. Dig. Endosc. 28, 186–193 (2015).
Günther, U. et al. Diagnostic value of confocal endomicroscopy in celiac disease. Endoscopy 42, 197–202 (2010).
Boschetto, D., Mirzaei, H., Leong, R., Tarroni, G. & Grisan, E. Semiautomatic detection of villi in confocal endoscopy for the evaluation of celiac disease. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 8143–8146 (2015).
Boschetto, D., Mirzaei, H., Leong, R. & Grisan, E. Detection and density estimation of goblet cells in confocal endoscopy for the evaluation of celiac disease. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 6248–6251 (2015).
Venkatesh, K. et al. Feasibility of confocal endomicroscopy in the diagnosis of pediatric gastrointestinal disorders. World J. Gastroenterol. 15, 2214–2219 (2009).
Kiesslich, R. et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 133, 1769–1778 (2007).
Turcotte, J.-F. et al. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest. Endosc. 77, 624–630 (2013).
Fritscher-Ravens, A. et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 147, 1012–1020.e4 (2014).
Lim, L. G. et al. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 20, 892–900 (2014).
Tabatabaei, N. et al. Tethered confocal endomicroscopy capsule for diagnosis and monitoring of eosinophilic esophagitis. Biomed. Opt. Express 5, 197–207 (2014).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Yang, V. X. et al. Endoscopic Doppler optical coherence tomography in the human GI tract: initial experience. Gastrointest. Endosc. 61, 879–890 (2005).
Hamdan, R., Gonzalez, R. G., Ghostine, S. & Caussin, C. Optical coherence tomography: from physical principles to clinical applications. Arch. Cardiovasc. Dis. 105, 529–534 (2012).
Wang, S. et al. Direct four-dimensional structural and functional imaging of cardiovascular dynamics in mouse embryos with 1.5 MHz optical coherence tomography. Opt. Lett. 40, 4791–4794 (2015).
Jung, W. et al. Three-dimensional endoscopic optical coherence tomography by use of a two-axis microelectromechanical scanning mirror. Appl. Phys. Lett. 88, 163901 (2006).
Sergeev, A. et al. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt. Express 1, 432–440 (1997).
Suter, M. J. et al. Esophageal-guided biopsy with volumetric laser endomicroscopy and laser cautery marking: a pilot clinical study. Gastrointest. Endosc. 79, 886–896 (2014).
Jang, I.-K. et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 111, 1551–1555 (2005).
Bouma, B. E., Tearney, G. J., Compton, C. C. & Nishioka, N. S. High-resolution imaging of the human esophagus and stomach in vivo using optical coherence tomography. Gastrointest. Endosc. 51, 467–474 (2000).
Gora, M. J. et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat. Med. 19, 238–240 (2013).
Wolfsen, H. C. et al. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett's esophagus (with videos). Gastrointest. Endosc. 82, 631–640 (2015).
Gora, M. J. et al. Tethered capsule endomicroscopy for comprehensive imaging of the human small intestine. Proc. SPIE, 9304, 9304–214 (2016).
Masci, E. et al. Optical coherence tomography in pediatric patients: a feasible technique for diagnosing celiac disease in children with villous atrophy. Dig. Liver Dis. 41, 639–643 (2009).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy (Springer, 2006).
Masters, B. R. & Chance, B. in Fluorescent and Luminescent Probes for Biological Activity 2nd edn Ch. 28 (ed. Mason, W. T. ) (Elsevier, 1999).
Skala, M. C. et al. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia. J. Biomed. Opt. 12, 024014 (2007).
Walsh, A. J. & Skala, M. C. Optical metabolic imaging quantifies heterogeneous cell populations. Biomed. Opt. Express 6, 559–573 (2015).
Wang, H. W. et al. Diffuse reflectance spectroscopy detects increased hemoglobin concentration and decreased oxygenation during colon carcinogenesis from normal to malignant tumors. Opt. Express 17, 2805–2817 (2009).
Douplik, A. et al. Diffuse reflectance spectroscopy in Barrett's esophagus: developing a large field-of-view screening method discriminating dysplasia from metaplasia. J. Biophotonics 7, 304–311 (2014).
Bigio, I. J. & Bown, S. G. Spectroscopic sensing of cancer and cancer therapy: current status of translational research. Cancer Biol. Ther. 3, 259–267 (2004).
Backman, V. et al. Detection of preinvasive cancer cells. Nature 406, 35–36 (2000).
Qiu, L. et al. Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett's esophagus. Nat. Med. 16, 603–606 (2010).
Wallace, M. B. et al. Endoscopic detection of dysplasia in patients with Barrett's esophagus using light-scattering spectroscopy. Gastroenterology 119, 677–682 (2000).
Mutyal, N. N. et al. In vivo risk analysis of pancreatic cancer through optical characterization of duodenal mucosa. Pancreas 44, 735–741 (2015).
Westrom, B. R., Svendsen, J., Ohlsson, B. G., Tagesson, C. & Karlsson, B. W. Intestinal transmission of macromolecules (BSA and FITC-labelled dextrans) in the neonatal pig. Influence of age of piglet and molecular weight of markers. Biol. Neonate 46, 20–26 (1984).
Ekstrom, G. M., Westrom, B. R., Telemo, E. & Karlsson, B. W. The uptake of fluorescein-conjugated dextran 70,000 by the small intestinal epithelium of the young rat and pig in relation to macromolecular transmission into the blood. J. Dev. Physiol. 10, 227–233 (1988).
Hodges, G. M., Carr, E. A., Hazzard, R. A. & Carr, K. E. Uptake and translocation of microparticles in small intestine. Morphology and quantification of particle distribution. Dig. Dis. Sci. 40, 967–975 (1995).
Limpanussorn, J., Simon, L. & Dayan, A. D. Transepithelial transport of large particles in rat: a new model for the quantitative study of particle uptake. J. Pharm. Pharmacol. 50, 753–760 (1998).
Hillyer, J. F. & Albrecht, R. M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 90, 1927–1936 (2001).
McCullough, J. S., Hodges, G. M., Dickson, G. R., Yarwood, A. & Carr, K. E. A morphological and microanalytical investigation into the uptake of particulate iron across the gastrointestinal tract of rats. J. Submicrosc. Cytol. Pathol. 27, 119–124 (1995).
Bell, A. G. The photophone. Science 1, 130–134 (1880).
Cox, B., Laufer, J. G., Arridge, S. R. & Beard, P. C. Quantitative spectroscopic photoacoustic imaging: a review. J. Biomed. Opt. 17, 061202 (2012).
Zackrisson, S., van de Ven, S. M. W. Y. & Gambhir, S. S. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res. 74, 979–1004 (2014).
Colchester, R. J. et al. Broadband miniature optical ultrasound probe for high resolution vascular tissue imaging. Biomed. Opt. Express 6, 1502–1511 (2015).
Yang, J. M. et al. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med. 18, 1297–1302 (2012).
Yuan, Y., Yang, S. & Xing, D. Preclinical photoacoustic imaging endoscope based on acousto-optic coaxial system using ring transducer array. Opt. Lett. 35, 2266–2268 (2010).
Tang, J. et al. Noninvasive high-speed photoacoustic tomography of cerebral hemodynamics in awake-moving rats. J. Cereb. Blood Flow Metab. 35, 1224–1232 (2015).
Hennen, S. N. et al. Photoacoustic tomography imaging and estimation of oxygen saturation of hemoglobin in ocular tissue of rabbits. Exp. Eye Res. 138, 153–158 (2015).
Mankins, J. C. Technological readiness levels: a white paper (NASA, 1995).
Kastanos, E. K., Kyriakides, A., Hadjigeorgiou, K. & Pitris, C. A novel method for urinary tract infection diagnosis and antibiogram using Raman spectroscopy. J. Raman Spectrosc. 41, 958–963 (2010).
de Siqueira e Oliveira, F. S., Giana, H. E. & Silveira, L. Discrimination of selected species of pathogenic bacteria using near-infrared Raman spectroscopy and principal components analysis. J. Biomed. Opt. 17, 107004 (2012).
Matysiak-Budnik, T. et al. In vivo real-time imaging of human duodenal mucosal structures in celiac disease using endocytoscopy. Endoscopy 42, 191–196 (2010).
Liu, G. Z., Qi, M., Hutchinson, M. R., Yang, G. F. & Goldys, E. M. Recent advances in cytokine detection by immunosensing. Biosens. Bioelectron. 79, 810–821 (2016).
Acknowledgements
The authors acknowledge funding from the Bill & Melinda Gates Foundation under the Virtual Biopsy of the Gut project (Opportunity ID: OPP1127324) and from the UK Engineering and Physical Sciences Research Council (Smart Sensing for Surgery, EP/L014149/1).
Author information
Authors and Affiliations
Contributions
A.J.T., M.H., S.A., L.S.C., T.T., C.L., W.A.F., T.J.M., R.R.-K. and P.K. researched data for the article. A.J.T., M.H., S.A., L.S.C., T.T., P.D., F.L., S.L., A.M., R.R.-K., G.J.T., P.K. and G.-Z.Y. discussed content for the article. A.J.T., M.H., S.A., L.S.C., T.T., C.L., W.A.F., T.J.M., A.M., R.R.-K., G.J.T., and P.K. wrote the manuscript. A.J.T., M.H., S.A., G.J.T., P.K. and G.-Z.Y. reviewed/edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
Massachusetts General Hospital, Massachusetts, USA, has a licensing arrangement with NinePoint Medical, and G.J.T. has the rights to receive royalties from this licensing arrangement. G.J.T. consults for NinePoint Medical. G.J.T. receives industry sponsored research funding from Ardea Biosciences, Canon, DL Biotech and iLumen Medical. T.J.M. is Chairman of the Board of NinePoint Medical and is compensated in stock for this activity. F.L. and S.L. are employed by and own stock in Mauna Kea Technologies. P.D. is employed by and owns stock in Optiscan Imaging. T.T. is employed by Olympus UK, a manufacturer of standard and capsule endoscopes for medical applications.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thompson, A., Hughes, M., Anastasova, S. et al. The potential role of optical biopsy in the study and diagnosis of environmental enteric dysfunction. Nat Rev Gastroenterol Hepatol 14, 727–738 (2017). https://doi.org/10.1038/nrgastro.2017.147
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.147
This article is cited by
-
Environmental enteric dysfunction: gut and microbiota adaptation in pregnancy and infancy
Nature Reviews Gastroenterology & Hepatology (2023)
-
Transcutaneous fluorescence spectroscopy as a tool for non-invasive monitoring of gut function: first clinical experiences
Scientific Reports (2020)
-
Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies
BMC Medicine (2019)
-
Gastrointestinal diagnosis using non-white light imaging capsule endoscopy
Nature Reviews Gastroenterology & Hepatology (2019)
-
Modular Robotic Scanning Device for Real-Time Gastric Endomicroscopy
Annals of Biomedical Engineering (2019)