Key Points
-
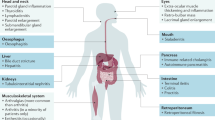
IgG4-related hepatobiliary diseases (IgG4-HBD) are part of a systemic fibroinflammatory condition, termed IgG4-related disease. IgG4-HBD includes IgG4-related sclerosing cholangitis (IgG4-SC) and IgG4-related hepatopathy
-
IgG4-HBD can present with abnormal liver biochemistry, biliary strictures and/or masses, impeding the differentiation from other benign and malignant hepatobiliary disorders
-
Diagnosis is based on a combination of clinical, biochemical, radiological and histological findings. A gold standard diagnostic test is lacking, although novel markers might help to differentiate IgG4-HBD from other conditions
-
Treatment regimens have been reached by international consensus but are not supported by randomized controlled trials. First-line therapy is corticosteroids, often in combination with biliary stenting for patients with IgG4-SC
-
The long-term outcome in IgG4-HBD is not well established. Disease-related inflammatory and fibrotic complications and an increased risk of all-cause malignancy have been reported in prospective studies
-
Insights into the genetic and immunological aspects of disease pathogenesis have increased over the past decade. Defining the initiating and driving factors for fibrotic disease remains an important challenge
Abstract
IgG4-related hepatobiliary diseases are part of a multiorgan fibroinflammatory condition termed IgG4-related disease, and include IgG4-related sclerosing cholangitis (IgG4-SC) and IgG4-related hepatopathy. These diseases can present with biliary strictures and/or mass lesions, making them difficult to differentiate from primary sclerosing cholangitis (PSC) or other hepatobiliary malignancies. Diagnosis is based on a combination of clinical, biochemical, radiological and histological findings. However, a gold standard diagnostic test is lacking, warranting the identification of more specific disease markers. Novel assays — such as the serum IgG4:IgG1 ratio and IgG4:IgG RNA ratio (which distinguish IgG4-SC from PSC with high serum IgG4 levels), and plasmablast expansion to recognize IgG4-SC with normal serum IgG4 levels — require further validation. Steroids and other immunosuppressive therapies can lead to clinical and radiological improvement when given in the inflammatory phase of the disease, but evidence for the efficacy of treatment regimens is limited. Progressive fibrosclerotic disease, liver cirrhosis and an increased risk of malignancy are now recognized outcomes. Insights into the genetic and immunological features of the disease have increased over the past decade, with an emphasis on HLAs, T cells, circulating memory B cells and plasmablasts, chemokine-mediated trafficking, as well as the role of the innate immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stone, J. H. IgG4-related disease: nomenclature, clinical features, and treatment. Semin. Diagn. Pathol. 29, 177–190 (2012).
Bartholomew, L. G., Cain, J. C., Woolner, L. B., Utz, D. C. & Ferris, D. O. Sclerosing cholangitis: its possible association with Riedel's struma and fibrous retroperitonitis. Report of two cases. N. Engl. J. Med. 269, 8–12 (1963).
Yoshida, K. et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig. Dis. Sci. 40, 1561–1568 (1995).
Hamano, H. et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N. Engl. J. Med. 344, 732–738 (2001).
Kamisawa, T., Egawa, N. & Nakajima, H. Autoimmune pancreatitis is a systemic autoimmune disease. Am. J. Gastroenterol. 98, 2811–2812 (2003).
Zen, Y., Nakanuma, Y. & Portmann, B. Immunoglobulin G4-related sclerosing cholangitis: pathologic features and histologic mimics. Semin. Diagn. Pathol. 29, 205–211 (2012).
Umemura, T. et al. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology 46, 463–471 (2007).
Umemura, T. et al. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut 56, 1471–1472 (2007).
Hamano, H. et al. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J. Gastroenterol. 41, 1197–1205 (2006).
Kanno, A. et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2011. Pancreas 44, 535–539 (2015).
Kanno, A. et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan. Pancreas 41, 835–839 (2012).
Tanaka, A. et al. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J. Hepatobiliary Pancreat. Sci. 21, 43–50 (2014).
Uchida, K., Masamune, A., Shimosegawa, T. & Okazaki, K. Prevalence of IgG4-Related Disease in Japan Based on Nationwide Survey in 2009. Int. J. Rheumatol. 2012, http://dx.doi.org/10.1155/2012/358371 (2012).
Ghazale, A. et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology 134, 706–715 (2008).
Huggett, M. T. et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am. J. Gastroenterol. 109, 1675–1683 (2014).
Nakanuma, Y., Tsuneyama, K., Masuda, S. & Tomioka, T. Hepatic inflammatory pseudotumor associated with chronic cholangitis: report of three cases. Hum. Pathol. 25, 86–91 (1994).
de Buy Wenniger, L. J. M., Culver, E. L. & Beuers, U. Exposure to occupational antigens might predispose to IgG4-related disease. Hepatology 60, 1453–1454 (2014).
Della Torre, E. et al. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy 69, 269–272 (2014).
Kamisawa, T., Anjiki, H., Egawa, N. & Kubota, N. Allergic manifestations in autoimmune pancreatitis. Eur. J. Gastroenterol. Hepatol. 21, 1136–1139 (2009).
Mattoo, H., Della-Torre, E., Mahajan, V. S., Stone, J. H. & Pillai, S. Circulating Th2 memory cells in IgG4- related disease are restricted to a defined subset of subjects with atopy. Allergy 69, 399–402 (2014).
Huggett, M. T. et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am. J. Gastroenterol. 109, 1675–1683 (2014).
Oh, H.-C. et al. Clinical clues to suspicion of IgG4-associated sclerosing cholangitis disguised as primary sclerosing cholangitis or hilar cholangiocarcinoma. J. Gastroenterol. Hepatol. 25, 1831–1837 (2010).
Joshi, D. & Webster, G. J. M. Review article: Biliary and hepatic involvement in IgG4-related disease. Aliment. Pharmacol. Ther. 40, 1251–1261 (2014).
Culver, E. L. et al. Increased IgG4 responses to multiple food and animal antigens indicate a polyclonal expansion and differentiation of pre-existing B cells in IgG4-related disease. Ann. Rheum. Dis. 74, 944–947 (2015).
Sah, R. P. & Chari, S. T. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr. Opin. Rheumatol. 23, 108–113 (2011).
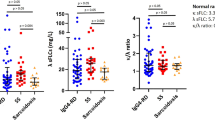
Boonstra, K. et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 59, 1954–1963 (2014).
Mendes, F. D. et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am. J. Gastroenterol. 101, 2070–2075 (2006).
Carruthers, M. N., Khosroshahi, A., Augustin, T., Deshpande, V. & Stone, J. H. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann. Rheum. Dis. 74, 14–18 (2015).
Oseini, A. M. et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 54, 940–948 (2011).
Ohara, H. et al. Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related sclerosing cholangitis: A Japanese cohort. J. Gastroenterol. Hepatol. 28, 1247–1251 (2013).
Nakazawa, T. et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest. Endosc. 60, 937–944 (2004).
Nakazawa, T., Ohara, H., Sano, H., Ando, T. & Joh, T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas 32, 229 (2006).
Culver, E. L. et al. Elevated serum IgG4 levels in diagnosis, treatment response, organ involvement and relapse in a prospective IgG4-related disease UK cohort. Am. J. Gastroenterol. 111, 733–743 (2016).
Doorenspleet, M. E. et al. IgG4+ B-cell receptor clones distinguish IgG4-related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology 64, 501–507 (2016).
Mattoo, H. et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J. Allergy Clin. Immunol. 134, 679–687 (2014).
Wallace, Z. S. et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann. Rheum. Dis. 74, 190–195 (2015).
Kerkman, P. F. et al. Circulating plasmablasts/plasmacells as a source of anticitrullinated protein antibodies in patients with rheumatoid arthritis. Ann. Rheum. Dis. 72, 1259–1263 (2013).
Jacobi, A. M. et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 48, 1332–1342 (2003).
Culver, E. L. et al. Immunoglobulin E, eosinophils and mast cells in atopic individuals provide novel insights in IgG4-related disease. J. Hepatol. 64 (Suppl. 2), S646 (2016).
Koyama, R. et al. Ultrasonographic imaging of bile duct lesions in autoimmune pancreatitis. Pancreas 37, 259–264 (2008).
Itoh, S. et al. Lymphoplasmacytic sclerosing cholangitis: assessment of clinical, CT, and pathological findings. Clin. Radiol. 64, 1104–1114 (2009).
Ohara, H. et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J. Hepatobiliary Pancreat. Sci. 19, 536–542 (2012).
Tokala, A., Khalili, K., Menezes, R., Hirschfield, G. & Jhaveri, K. S. Comparative MRI analysis of morphologic patterns of bile duct disease in IgG4-related systemic disease versus primary sclerosing cholangitis. AJR. Am. J. Roentgenol. 202, 536–543 (2014).
Naitoh, I. et al. Small bile duct involvement in IgG4-related sclerosing cholangitis: Liver biopsy and cholangiography correlation. J. Gastroenterol. 46, 269–276 (2011).
Zhang, J. et al. Characterizing IgG4-related disease with 18F-FDG PET/CT: a prospective cohort study. Eur. J. Nucl. Med. Mol. Imaging 41, 1624–1634 (2014).
Tabata, T. et al. Differentiating immunoglobulin g4-related sclerosing cholangitis from hilar cholangiocarcinoma. Gut Liver 7, 234–238 (2013).
Kalaitzakis, E. et al. Endoscopic retrograde cholangiography does not reliably distinguish IgG4-associated cholangitis from primary sclerosing cholangitis or cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 9, 800–803.e2 (2011).
Kawakami, H. & Zen, Y. Is IgG4 immunostaining of duodenal ampullary biopsies alone useful to diagnose autoimmune pancreatitis? Gastrointest. Endosc. 72, 1328; author reply 1328–1329 (2010).
Okano, N., Igarashi, Y., Kishimoto, Y., Ito, K., Sasai, D. Case of immunoglobulin G4-related cholangitis accompanying autoimmune pancreatitis: diagnosis by peroral cholangioscopy and treatment by endoscopic biliary stenting. Dig. Endosc. 24, 62–66 (2012).
Nakazawa, T., Naitoh, I. & Hayashi, K. Usefulness of intraductal ultrasonography in the diagnosis of cholangiocarcinoma and IgG4-related sclerosing cholangitis. Clin. Endosc. 45, 331 (2012).
Naitoh, I. et al. Predictive factors for positive diagnosis of malignant biliary strictures by transpapillary brush cytology and forceps biopsy. J. Dig. Dis. 17, 44–51 (2016).
Itoi, T. et al. Diagnostic peroral video cholangioscopy is an accurate diagnostic tool for patients with bile duct lesions. Clin. Gastroenterol. Hepatol. 8, 934–938 (2010).
Moon, S.-H. et al. IgG4 immunostaining of duodenal papillary biopsy specimens may be useful for supporting a diagnosis of autoimmune pancreatitis. Gastrointest. Endosc. 71, 960–966 (2010).
Cebe, K. M., Swanson, P. E., Upton, M. P. & Westerhoff, M. Increased IgG4+ cells in duodenal biopsies are not specific for autoimmune pancreatitis. Am. J. Clin. Pathol. 139, 323–329 (2013).
Vosskuhl, K. et al. Measurement of IgG4 in bile: A new approach for the diagnosis of IgG4-associated cholangiopathy. Endoscopy 44, 48–52 (2012).
Stone, J. H. J. R. et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 64, 3061–3067 (2012).
Deshpande, V. et al. IgG4-associated cholangitis: a comparative histological and immunophenotypic study with primary sclerosing cholangitis on liver biopsy material. Mod. Pathol. 22, 1287–1295 (2009).
Culver, E. L. & Bateman, A. C. IgG4-related disease: can non-classical histopathological features or the examination of clinically uninvolved tissues be helpful in the diagnosis? J. Clin. Pathol. 65, 963–969 (2012).
Naitoh, I. et al. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J. Gastroenterol. 44, 1147–1155 (2009).
Deshpande, V. et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 25, 1181–1192 (2012).
Zhang, L. et al. IgG4+ plasma cell infiltrates in liver explants with primary sclerosing cholangitis. Am. J. Surg. Pathol. 34, 88–94 (2010).
Zen, Y., Quaglia, A. & Portmann, B. Immunoglobulin G4-positive plasma cell infiltration in explanted livers for primary sclerosing cholangitis. Histopathology 58, 414–422 (2011).
Harada, K. et al. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology 56, 157–164 (2012).
Strehl, J. D., Hartmann, A. & Agaimy, A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J. Clin. Pathol. 64, 237–243 (2011).
Zen, Y., Fujii, T., Sato, Y., Masuda, S. & Nakanuma, Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod. Pathol. 20, 884–894 (2007).
Chung, H. et al. Identification and characterization of IgG4-associated autoimmune hepatitis. Liver Int. 30, 222–231 (2010).
Nakazawa, T. et al. Diagnosis of IgG4-related sclerosing cholangitis. World J. Gastroenterol. 19, 7661–7670 (2013).
Chari, S. T. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J. Gastroenterol. 42 (Suppl. 1), 39–41 (2007).
Khosroshahi, A. et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 67, 1688–1699 (2015).
Kamisawa, T. et al. Standard steroid treatment for autoimmune pancreatitis. Gut 58, 1504–1507 (2009).
Buijs, J. et al. Comparable efficacy of low- versus high-dose induction corticosteroid treatment in autoimmune pancreatitis. Pancreas 43, 261–267 (2014).
Moon, S.-H. et al. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut 57, 1704–1712 (2008).
Hart, P. A. et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 62, 1771–1776 (2013).
Sandanayake, N. S. et al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin. Gastroenterol. Hepatol. 7, 1089–1096 (2009).
Hart, P. A. et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut 62, 1607–1615 (2013).
Topazian, M. et al. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin. Gastroenterol. Hepatol. 6, 364–366 (2008).
Carruthers, M. N. et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann. Rheum. Dis. 74, 1171–1177 (2015).
Takuma, K. et al. Short-term and long-term outcomes of autoimmune pancreatitis. Eur. J. Gastroenterol. Hepatol. 23, 146–152 (2011).
Yamamoto, M. et al. Risk of malignancies in IgG4-related disease. Mod. Rheumatol. 22, 414–418 (2012).
Aalberse, R. C., van der Gaag, R. & van Leeuwen, J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J. Immunol. 130, 722–726 (1983).
Aalberse, R. C., Stapel, S. O., Schuurman, J. & Rispens, T. Immunoglobulin G4: an odd antibody. Clin. Exp. Allergy 39, 469–477 (2009).
Kawa, S. et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology 122, 1264–1269 (2002).
Park, D. H. et al. Substitution of aspartic acid at position 57 of the DQβ1 affects relapse of autoimmune pancreatitis. Gastroenterology 134, 440–446 (2008).
Hirano, K. et al. No significant relation between relapse of autoimmune pancreatitis and substitution of aspartic acid at position 57 of DQβ1. J. Gastroenterol. 44, 799–800 (2009).
Culver, E. L. et al. Human leucocyte antigen associations in IgG4-related disease and primary sclerosing cholangitis stratified by IgG4 levels, in a multicenter UK cohort. J. Hepatol. 64, S646 (2016).
Umemura, T. et al. Association of autoimmune pancreatitis with cytotoxic T-lymphocyte antigen 4 gene polymorphisms in Japanese patients. Am. J. Gastroenterol. 103, 588–594 (2008).
Umemura, T. et al. Genetic association of Fc receptor-like 3 polymorphisms with autoimmune pancreatitis in Japanese patients. Gut 55, 1367–1368 (2006).
Chang, M.-C. et al. T-Cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin. Chem. 53, 1700–1705 (2007).
Maillette de Buy Wenniger, L. J. et al. Immunoglobulin G4+ clones identified by next-generation sequencing dominate the B cell receptor repertoire in immunoglobulin G4 associated cholangitis. Hepatology 57, 2390–2398 (2013).
Asada, M. et al. Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 33, 20–26 (2006).
Aparisi, L. et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut 54, 703–709 (2005).
Nishimori, I. et al. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut 54, 274–281 (2005).
Löhr, J.-M. et al. Autoantibodies against the exocrine pancreas in autoimmune pancreatitis: gene and protein expression profiling and immunoassays identify pancreatic enzymes as a major target of the inflammatory process. Am. J. Gastroenterol. 105, 2060–2071 (2010).
Guarneri, F., Guarneri, C. & Benvenga, S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J. Cell. Mol. Med. 9, 741–744 (2005).
Kountouras, J., Zavos, C. & Chatzopoulos, D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J. Cell. Mol. Med. 9, 196–207 (2005).
Frulloni, L. et al. Identification of a novel antibody associated with autoimmune pancreatitis. N. Engl. J. Med. 361, 2135–2142 (2009).
Jesnowski, R. et al. Helicobacter pylori in autoimmune pancreatitis and pancreatic carcinoma. Pancreatology 10, 462–466 (2010).
Culver, E. L. et al. Helicobacter Pylori as a microbial antigen in IgG4-related disease. J. Hepatol. 64, S644 (2016).
Aoki, S. et al. Immunohistochemical study of autoimmune pancreatitis using anti-IgG4 antibody and patients' sera. Histopathology 47, 147–158 (2005).
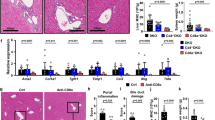
Shiokawa, M. et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut 65, 1322–1332 (2016).
Zen, Y. et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 45, 1538–1546 (2007).
Müller, T. et al. Increased T-helper 2 cytokines in bile from patients with IgG4-related cholangitis disrupt the tight junction–associated biliary epithelial cell barrier. Gastroenterology 144, 1116–1128 (2013).
Zen, Y. et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 45, 1538–1546 (2007).
Tanaka, A. et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 64, 254–263 (2012).
Kanari, H. et al. Role of Th2 cells in IgG4-related lacrimal gland enlargement. Int. Arch. Allergy Immunol. 152 (Suppl. 1), 47–53 (2010).
Takeuchi, M. et al. T helper 2 and regulatory T-cell cytokine production by mast cells: a key factor in the pathogenesis of IgG4-related disease. Mod. Pathol. 27, 1126–1136 (2014).
Takeuchi, M. et al. Interleukin 13-positive mast cells are increased in immunoglobulin G4-related sialadenitis. Sci. Rep. 5, http://dx.doi.org/10.1038/srep07696 (2015).
Tsuboi, H. et al. Analysis of IgG4 class switch-related molecules in IgG4-related disease. Arthritis Res. Ther. 14, http://dx.doi.org/10.1186/ar3924 (2012).
Miyoshi, H. et al. Circulating naïve and CD4+CD25high regulatory T cells in patients with autoimmune pancreatitis. Pancreas 36, 133–140 (2008).
Kusuda, T. et al. Involvement of inducible costimulator- and interleukin 10-positive regulatory T cells in the development of IgG4-related autoimmune pancreatitis. Pancreas 40, 1120–1130 (2011).
Uchida, K. et al. Regulatory T cells in type 1 autoimmune pancreatitis. Int. J. Rheumatol. 2012, http://dx.doi.org/10.1155/2012/795026 (2012).
Akiyama, M. et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol. 67, 2476–2481 (2015).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2012).
Esposito, I. et al. Autoimmune pancreatocholangitis, non-autoimmune pancreatitis and primary sclerosing cholangitis: a comparative morphological and immunological analysis. PLoS ONE 3, http://dx.doi.org/10.1371/journal.pone.0002539 (2008).
Mattoo, H. et al. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2015.12.1330 (2016).
Lighaam, L. C. et al. Phenotypic differences between IgG4+ and IgG1+ B cells point to distinct regulation of the IgG4 response. J. Allergy Clin. Immunol. 133, 267–270. e1–6 (2014).
Graham, R. P. D., Smyrk, T. C., Chari, S. T., Takahashi, N. & Zhang, L. Isolated IgG4-related sclerosing cholangitis: a report of 9 cases. Hum. Pathol. 45, 1722–1729 (2014).
Zen, Y., Liberal, R., Nakanuma, Y., Heaton, N. & Portmann, B. Possible involvement of CCL1-CCR8 interaction in lymphocytic recruitment in IgG4-related sclerosing cholangitis. J. Hepatol. 59, 1059–1064 (2013).
Seleznik, G. M. et al. Lymphotoxin β receptor signaling promotes development of autoimmune pancreatitis. Gastroenterology 143, 1361–1374 (2012).
Culver, E. L. et al. Gene expression analysis identifies immune signaling and complment pathways in IgG4-related disease. J. Hepatol. 60, S185–S186 (2014).
Akitake, R. et al. Possible involvement of T helper type 2 responses to Toll-like receptor ligands in IgG4-related sclerosing disease. Gut 59, 542–545 (2010).
Watanabe, T. et al. Toll-like receptor activation in basophils contributes to the development of IgG4-related disease. J. Gastroenterol. 48, 247–253 (2013).
Furukawa, S. et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Clin. Immunol. 156, 9–18 (2015).
Khosroshahi, A., Bloch, D. B., Deshpande, V. & Stone, J. H. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 62, 1755–1762 (2010).
Khosroshahi, A. et al. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 91, 57–66 (2012).
Carruthers, M. N. et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann. Rheum. Dis. 74, 1171–1177 (2015).
Wallace, Z. S. et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann. Rheum. Dis. 74, 190–195 (2015).
Della-Torre, E. et al. B-Cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann. Rheum. Dis. 74, 2236–2243 (2015).
Nagata, K. et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s). FEBS Lett. 459, 195–199 (1999).
Saito, Y. et al. Roles of CRTH2+ CD4+ T cells in immunoglobulin G4-related lacrimal gland enlargement. Int. Arch. Allergy Immunol. 158 (Suppl.), 42–46 (2012).
Huang, T. et al. Depletion of major pathogenic cells in asthma by targeting CRTh2. JCI Insight. 1, e86689 (2016).
Beuers, U., Boberg, K. M. & Chapman, R. W. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J. Hepatol. 51, 237–267 (2009).
Deshpande, V. et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 25, 1181–1192 (2012).
Lin, W. et al. Clinical characteristics of immunoglobulin G4-related disease: a prospective study of 118 Chinese patients. Rheumatology (Oxford) 54, 1982–1990 (2015).
Inoue, D. et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 94, http://dx.doi.org/10.1097/MD.0000000000000680 (2015).
Fernández-Codina, A. et al. IgG4-related disease: results from a multicenter Spanish registry. Medicine (Baltimore) 94, http://dx.doi.org/10.1097/MD.0000000000001275 (2015).
Campochiaro, C. et al. IgG4-related disease in Italy: clinical features and outcomes of a large cohort of patients. Scand. J. Rheumatol. 45, 135–145 (2015).
Nakanuma, Y. & Zen, Y. Pathology and immunopathology of immunoglobulin G4-related sclerosing cholangitis: The latest addition to the sclerosing cholangitis family. Hepatol. Res. 37 (Suppl. 3), S478–486 (2007).
Ravi, K. et al. Inflammatory bowel disease in the setting of autoimmune pancreatitis. Inflamm. Bowel Dis. 15, 1326–1330 (2009).
Navaneethan, U. & Shen, B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm. Bowel Dis. 16, 1598–1619 (2010).
Webster, G. J. M., Pereira, S. P. & Chapman, R. W. Autoimmune pancreatitis/IgG4-associated cholangitis and primary sclerosing cholangitis—overlapping or separate diseases? J. Hepatol. 51, 398–402 (2009).
Stinton, L. M. et al. PR3-ANCA: a promising biomarker in primary sclerosing cholangitis (PSC). PLoS ONE 9, http://dx.doi.org/10.1371/journal.pone.0112877 (2014).
Boonstra, K. et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 59, 1954–1963 (2014).
Berntsen, N. L. et al. Association between HLA haplotypes and increased serum levels of IgG4 in patients with primary sclerosing cholangitis. Gastroenterology 148, 924–927.e2 (2015).
Liaskou, E. & Hirschfield, G. M. Genetic distinctions in patients with primary sclerosing cholangitis: immunoglobulin G4 elevations and HLA risk. Gastroenterology 148, 886–889 (2015).
Gardner, C. S. et al. Diagnostic performance of imaging criteria for distinguishing autoimmune cholangiopathy from primary sclerosing cholangitis and bile duct malignancy. Abdom. Imaging 40, 3052–3061 (2015).
Ruemmele, P., Hofstaedter, F. & Gelbmann, C. M. Secondary sclerosing cholangitis. Nat. Rev. Gastroenterol. Hepatol. 6, 287–295 (2009).
Hirano, K. et al. Involvement of the biliary system in autoimmune pancreatitis: A follow-up study. Clin. Gastroenterol. Hepatol. 1, 453–464 (2003).
Erdogan, D. et al. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br. J. Surg. 95, 727–734 (2008).
Author information
Authors and Affiliations
Contributions
E.L.C. wrote the article, researched data and provided a substantial contribution to discussions of the content. E.L.C. and R.W.C. contributed equally to the reviewing and/or editing of the manuscript before submission
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (box)
IgG4 antibody structure and function (PDF 111 kb)
Supplementary information S2 (table)
Autoantibodies and/or autoantigens in AIP (and IgG4-SC) (PDF 121 kb)
Supplementary information S3 (table)
IgG4 autoantibodies and/or antigens in non-IgG4-RD (PDF 124 kb)
Rights and permissions
About this article
Cite this article
Culver, E., Chapman, R. IgG4-related hepatobiliary disease: an overview. Nat Rev Gastroenterol Hepatol 13, 601–612 (2016). https://doi.org/10.1038/nrgastro.2016.132
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.132
This article is cited by
-
An atypical case of isolated immunoglobulin G4-related sclerosing cholangitis with a cholangiogram resembling primary sclerosing cholangitis
Clinical Journal of Gastroenterology (2024)
-
IgG4-assoziierte Autoimmunerkrankungen
Die Gastroenterologie (2022)
-
Immunoglobulin G4-related autoimmune hepatitis simultaneously concomitant with autoimmune pancreatitis: a case report
Clinical Journal of Gastroenterology (2021)
-
Malignancy Risk of Immunoglobin G4-Related Disease: Evidence from a Large Cohort Multicenter Retrospective Study
Rheumatology and Therapy (2021)
-
IgG4-Related Hepatic Inflammatory Pseudotumor Misdiagnosed as Cholangiocellular Carcinoma of the Liver
Journal of Gastrointestinal Surgery (2021)