Key Points
-
Cancer screening regimens are recommended for liver transplant recipients to compensate for the increased risk of certain malignancies
-
Improved risk factor management is necessary to reduce and prevent cardiovascular and renal disease in the liver transplant recipient
-
Treatment of recurrent viral hepatitis can improve long-term graft and patient survival
-
Pregnancy after transplantation is considered a 'high-risk pregnancy' and therapeutic adjustments are necessary to accommodate pregnancy-related issues in transplant recipients
-
The use of oral contraceptives in liver transplant recipients depends on allograft function and certain drugs might be contraindicated
-
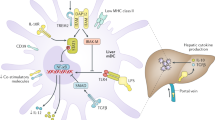
Transplant recipients are at a higher risk of acquiring infections than healthy individuals; vaccination is therefore highly recommended, ideally before immunosuppression is started
Abstract
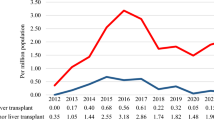
Improvements in overall survival early after liver transplantation result in a growing number of patients with the potential for long-term survival. Data available on long-term survival, to date, reflect the situation of patients who received their liver transplant during a very different health-care era. Translating these data into the current medical era of liver transplantation is an important task, as a better understanding of aspects associated with morbidity and mortality is fundamental in improving the long-term outcome of liver transplant recipients. Malignancy screening, optimal treatment of recurrent disease and adequate management of metabolic disease are crucial contributions to advance patient care. In this Review, data specific to the liver transplant recipient will be evaluated and, in the absence of sufficient evidence at this time, recommendations and guidelines for the general population on management of long-term concerns will be assessed for their applicability after liver transplantation. In addition, other preventive strategies relating to pregnancy, contraception and vaccination are reviewed in detail.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
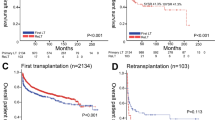
Agopian, V. G. et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann. Surg. 258, 409–421 (2013).
Allen, A. M. et al. Chronic kidney disease and associated mortality after liver transplantation—a time-dependent analysis using measured glomerular filtration rate. J. Hepatol. 61, 286–292 (2014).
McCaughan, G. W., Shackel, N. A., Strasser, S. I., Dilworth, P. & Tang, P. Minimal but significant improvement in survival for non-hepatitis C-related adult liver transplant patients beyond the one-year posttransplant mark. Liver Transpl. 16, 130–137 (2010).
Scientific Registry of Transplant Recipients [online], (2015).
Aberg, F. et al. Differences in long-term survival among liver transplant recipients and the general population: A population-based nordic study. Hepatology 61, 668–677 (2015).
Charlton, M. R. et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 141, 1249–1253 (2011).
Watt, K., Pedersen, R., Kremers, W., Heimbach, J. & Charlton, M. Evolution of causes and risk factors for mortality post liver transplant: results of the NIDDK Long Term Follow-up Study. Am. J. Transplant. 10, 1–8 (2010).
Charlton, M. et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 148, 108–117 (2015).
Kwo, P. Y. et al. An interferon-free antiviral regimen for HCV after liver transplantation. N. Engl. J. Med. 371, 2375–2382 (2014).
Burgio, M. D. et al. Follow-up imaging after liver transplantation should take into consideration primary hepatocellular carcinoma characteristics. Transplantation 99, 1613–1618 (2015).
Pecchi, A. et al. Post-transplantation hepatocellular carcinoma recurrence: patterns and relation between vascularity and differentiation degree. World J. Hepatol. 7, 276–284 (2015).
Aberg, F., Pukkala, E., Hockerstedt, K., Sankila, R. & Isoniemi, H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 14, 1428–1436 (2008).
Rothbaum Perito, E., Lau, A., Rhee, S., Roberts, J. P. & Rosenthal, P. Posttransplant metabolic syndrome in children and adolescents after liver transplantation: a systematic review. Liver Transpl. 18, 1009–1028 (2012).
Kelly, D. A. Current issues in pediatric transplantation. Pediatr. Transplant. 10, 712–720 (2006).
Pruthi, J. et al. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 7, 811–815 (2001).
Rabkin, J. M. et al. Late mortality after orthotopic liver transplantation. Am. J. Surg. 181, 475–479 (2001).
Fung, J. J. et al. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl. 7, S109–S118 (2001).
Schrem, H. et al. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 19, 1252–1261 (2013).
Watt, K., Pedersen, R., Heimbach, J., Kremers, W. & Gores, G. Probability and mortality of de novo malignancy after liver transplantation: results of the multicentered, prospective NIDDK long term database. Gastroenterology 137, 2010–2017 (2009).
Herrero, J. I. et al. Nonmelanoma skin cancer after liver transplantation. Study of risk factors. Liver Transpl. 11, 1100–1106 (2005).
Taylor, A. L., Marcus, R. & Bradley, J. A. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit. Rev. Oncol. Hematol. 56, 155–167 (2005).
Singh, S., Edakkanambeth Varayil, J., Loftus, E. V., Jr & Talwalkar, J. A. Incidence of colorectal cancer after liver transplantation for primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Transpl. 19, 1361–1369 (2013).
Razumilava, N., Gores, G. J. & Lindor, K. D. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 54, 1842–1852 (2011).
Lucey, M. R. et al. Long-Term Management of the Successful Adult Liver Transplant: 2012 Practice Guideline by AASLD and the American Society of Transplantation [online], (2012).
Fevery, J. et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int. 32, 214–222 (2012).
U.S. Preventive Services Task Force. Final Recommendation Statement Lung Cancer: Screening [online], (2013).
Church, T. R. et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 368, 1980–1991 (2013).
Herrero, J. I. et al. Lung cancer screening with low-radiation dose computed tomography after liver transplantation. Ann. Transplant. 18, 587–592 (2013).
Chandok, N. & Watt, K. D. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl. 18, 1277–1289 (2012).
Herrero, J. I. Screening of de novo tumors after liver transplantation. J. Gastroenterol. Hepatol. 27, 1011–1016 (2012).
Benlloch, S. et al. De novo internal neoplasms after liver transplantation: increased risk and aggressive behavior in recent years? Am. J. Transplant. 4, 596–604 (2004).
Finkenstedt, A. et al. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am. J. Transplant. 9, 2355–2361 (2009).
Krajewski, C. & Sucato, G. Reproductive health care after transplantation. Best Pract Res. Clin. Obstet. Gynaecol. 28, 1222–1234 (2014).
Madeleine, M. M., Finch, J. L., Lynch, C. F., Goodman, M. T. & Engels, E. A. HPV-related cancers after solid organ transplantation in the United States. Am. J. Transplant. 13, 3202–3209 (2013).
Na, R. et al. De novo cancer-related death in Australian liver and cardiothoracic transplant recipients. Am. J. Transplant. 13, 1296–1304 (2013).
Albeldawi, M. et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 18, 370–375 (2012).
Fussner, L. A. et al. Cardiovascular disease after liver transplantation. When, what and who is at risk. Liver Transpl. 21, 889–896 (2015).
Laish, I. et al. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 17, 15–22 (2011).
Bianchi, G., Marchesini, G., Marzocchi, R., Pinna, A. & Zoli, M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 4, 1648–1654 (2008).
Laryea, M. et al. Metabolic syndrome in liver transplant recipients: prevalence and association with cardiovascular events. Liver Transpl. 13, 1109–1114 (2007).
Garcia, A. M., Veneroso, C. E., Soares, D. D., Lima, A. S. & Correia, M. I. Effect of a physical exercise program on the functional capacity of liver transplant patients. Transplant. Proc. 46, 1807–1808 (2014).
Everhart, J. E. et al. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl. Surg. 4, 285–296 (1998).
Richards, J., Gunson, B., Johnson, J. & Neuberger, J. Weight gain and obesity after liver transplantation. Transpl. Int. 18, 461–466 (2005).
Anastacio, L. R. et al. Prospective evaluation of metabolic syndrome and its components among long-term liver recipients. Liver Int. 34, 1094–1101 (2014).
Cassiman, D. et al. Orlistat treatment is safe in overweight and obese liver transplant recipients: a prospective, open label trial. Transpl. Int. 19, 1000–1005 (2006).
Heimbach, J. K. et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am. J. Transplant. 13, 363–368 (2013).
Al-Nowaylati, A. R. et al. Gastric bypass after liver transplantation. Liver Transpl. 19, 1324–1329 (2013).
Lin, M. Y. et al. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg. Endosc. 27, 81–85 (2013).
Pageaux, G. P., Faure, S., Bouyabrine, H., Bismuth, M. & Assenat, E. Long-term outcomes of liver transplantation: diabetes mellitus. Liver Transpl. 15 (Suppl 2), S79–S82 (2009).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes care 35, 1364–1379 (2012).
Jenssen, T. & Hartmann, A. Emerging treatments for post-transplantation diabetes mellitus. Nat. Rev. Nephrol. 11, 465–477 (2015).
James, P. A. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520 (2014).
Textor, S. C., Taler, S. J., Canzanello, V. J., Schwartz, L. & Augustine, J. E. Posttransplantation hypertension related to calcineurin inhibitors. Liver Transpl. 6, 521–530 (2000).
Maggio, T. G. & Bartels, D. W. Increased cyclosporine blood concentrations due to verapamil administration. Drug Intell. Clin. Pharm. 22, 705–707 (1988).
Galioto, A. et al. Nifedipine versus carvedilol in the treatment of de novo arterial hypertension after liver transplantation: results of a controlled clinical trial. Liver Transpl. 14, 1020–1028 (2008).
Neal, D., Brown, M., Wilkinson, I., Byrne, C. & Alexander, G. Hemodynamic effects of amlodipine, bisoprolol, and lisinopril in hypertensive patients after liver transplantation. Transplantation 77, 748–750 (2004).
Abbas, G., Silveira, M. G. & Lindor, K. D. Hepatic fibrosis and the renin-angiotensin system. Am. J. Ther. 18, e202–e208 (2011).
Heinze, G. et al. Angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J. Am. Soc. Nephrol. 17, 889–899 (2006).
Hernandez, D. et al. Renin-angiotensin system blockade and kidney transplantation: a longitudinal cohort study. Nephrol. Dial. Transplant. 27, 417–422 (2012).
Ogata, T., Nomura, M., Nakaya, Y. & Ito, S. Evaluation of episodes of sleep apnea in patients with liver cirrhosis. J. Med. Invest. 53, 159–166 (2006).
Chhatrala, R. et al. Evolution of the serum atherogenic risk in liver transplant recipients; role of lipoproteins, metabolic and inflammatory markers. Liver Transpl. 21, 623–630 (2015).
Jellinger, P. et al. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr. Pract. 18, 1–78 (2012).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, 1–45 (2013).
Bays, H., Cohen, D. E., Chalasani, N., Harrison, S. A. & The National Lipid Association's Statin Safety Task, F. An assessment by the Statin Liver Safety Task Force: 2014 update. J. Clin. Lipidol. 8, 47–57 (2014).
Almutairi, F. et al. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 15, 504–508 (2009).
Brinton, E. A. Management of Hypertriglyceridemia for Prevention of Atherosclerotic Cardiovascular Disease. Cardiol. Clin. 33, 309–323 (2015).
Leidig-Bruckner, G. et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet 357, 342–347 (2001).
Pennisi, P. et al. Pamidronate and osteoporosis prevention in liver transplant recipients. Rheumatol. Int. 27, 251–256 (2007).
Guichelaar, M. M., Malinchoc, M., Sibonga, J., Clarke, B. L. & Hay, J. E. Immunosuppressive and postoperative effects of orthotopic liver transplantation on bone metabolism. Liver Transpl. 10, 638–647 (2004).
Westenfeld, R. et al. Impact of sirolimus, tacrolimus and mycophenolate mofetil on osteoclastogenesis—implications for post-transplantation bone disease. Nephrol. Dial. Transplant. 26, 4115–4123 (2011).
Millonig, G. et al. Alendronate in combination with calcium and vitamin D prevents bone loss after orthotopic liver transplantation: a prospective single-center study. Liver Transpl. 11, 960–966 (2005).
Cantarovich, M. et al. Early changes in kidney function predict long-term chronic kidney disease and mortality in patients after liver transplantation. Transplantation 92, 1358–1363 (2011).
Klintmalm, G. B. & Nashan, B. The role of mTOR inhibitors in liver transplantation: reviewing the evidence. J. Transplant 2014, 845438 (2014).
Blume, C., Pischke, S., von Versen-Hoynck, F., Gunter, H. H. & Gross, M. M. Pregnancies in liver and kidney transplant recipients: a review of the current literature and recommendation. Best Pract. Res. Clin. Obstet. Gynaecol. 28, 1123–1136 (2014).
Park-Wyllie, L. et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 62, 385–392 (2000).
Jain, A. et al. Pregnancy after liver transplantation under tacrolimus. Transplantation 64, 559–565 (1997).
Armenti, V. T. et al. National transplantation Pregnancy Registry—outcomes of 154 pregnancies in cyclosporine-treated female kidney transplant recipients. Transplantation 57, 502–506 (1994).
Kainz, A., Harabacz, I., Cowlrick, I. S., Gadgil, S. D. & Hagiwara, D. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation 70, 1718–1721 (2000).
Hoeltzenbein, M. et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am. J. Med. Genet. A 158A, 588–596 (2012).
Constantinescu, S. et al. Breast-feeding after transplantation. Best Pract. Res. Clin. Obstet. Gynaecol. 28, 1163–1173 (2014).
World Health Organization. Human Reproduction Programme. Medical eligibility criteria for contraceptive use. Fourth Edition [online], (2015).
Curtis, K. M., Jamieson, D. J., Peterson, H. B. & Marchbanks, P. A. Adaptation of the World Health Organization's medical eligibility criteria for contraceptive use for use in the United States. Contraception 82, 3–9 (2010).
Paulen, M. E., Folger, S. G., Curtis, K. M. & Jamieson, D. J. Contraceptive use among solid organ transplant patients: a systematic review. Contraception 82, 102–112 (2010).
U.S. Preventive Services Task Force. Strength of evidence grading system [online], (2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Watt, K. Keys to long-term care of the liver transplant recipient. Nat Rev Gastroenterol Hepatol 12, 639–648 (2015). https://doi.org/10.1038/nrgastro.2015.172
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.172
This article is cited by
-
Pre-transplant Cardiovascular Risk Assessment and Modification
Current Treatment Options in Gastroenterology (2022)
-
Impact of Allograft Steatosis on Cardiovascular Outcomes
Current Transplantation Reports (2018)