Abstract
The monolayer of columnar epithelial cells lining the gastrointestinal tract—the intestinal epithelial barrier (IEB)—is the largest exchange surface between the body and the external environment. The permeability of the IEB has a central role in the regulation of fluid and nutrient intake as well as in the control of the passage of pathogens. The functions of the IEB are highly regulated by luminal as well as internal components, such as bacteria or immune cells, respectively. Evidence indicates that two cell types of the enteric nervous system (ENS), namely enteric neurons and enteric glial cells, are potent modulators of IEB functions, giving rise to the novel concept of a digestive 'neuronal–glial–epithelial unit' akin to the neuronal–glial–endothelial unit in the brain. In this Review, we summarize findings demonstrating that the ENS is a key regulator of IEB function and is actively involved in pathologies associated with altered barrier function.
Key Points
-
Altered functioning of the intestinal epithelial barrier (IEB) has a central role in the aetiology of a wide range of diseases; efficient IEB healing is essential to maintain IEB homeostasis
-
An anatomical unit comprised of enteric neurons, enteric glial cells and intestinal epithelial cells sets the basis for a functional digestive neuronal–glial–epithelial unit
-
Enteric neuromediators as well as gliomediators can differentially modulate major IEB functions such as paracellular permeability, intestinal epithelial cell proliferation and wound healing
-
Changes in the phenotype of enteric neurons and glial cells occur in various diseases but the involvement of the enteric nervous system (active or bystander) in these pathologies remains to be defined
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sancho, E., Batlle, E. & Clevers, H. Live and let die in the intestinal epithelium. Curr. Opin. Cell Biol. 15, 763–770 (2003).
Blikslager, A. T., Moeser, A. J., Gookin, J. L., Jones, S. L. & Odle, J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 87, 545–564 (2007).
Taupin, D. & Podolsky, D. K. Trefoil factors: initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 4, 721–732 (2003).
Sturm, A. & Dignass, A. U. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 14, 348–353 (2008).
Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 32, 256–264 (2011).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Severson, E. A. & Parkos, C. A. Mechanisms of outside-in signaling at the tight junction by junctional adhesion molecule A. Ann. NY Acad. Sci. 1165, 10–18 (2009).
Vetrano, S. & Danese, S. The role of JAM-A in inflammatory bowel disease: unrevealing the ties that bind. Ann. NY Acad. Sci. 1165, 308–313 (2009).
Capaldo, C. T. & Macara, I. G. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 18, 189–200 (2007).
Van Itallie, C. M. et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 121, 298–305 (2008).
Watson, C. J., Hoare, C. J., Garrod, D. R., Carlson, G. L. & Warhurst, G. Interferon-γ selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J. Cell Sci. 118, 5221–5230 (2005).
Watson, C. J., Rowland, M. & Warhurst, G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiology. Cell Physiol. 281, C388–C397 (2001).
Anderson, J. M. & Van Itallie, C. M. Tight junctions. Curr. Biol. 18, R941–R943 (2008).
Artursson, P., Ungell, A. L. & Lofroth, J. E. Selective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Pharm. Res. 10, 1123–1129 (1993).
Knipp, G. T., Ho, N. F., Barsuhn, C. L. & Borchardt, R. T. Paracellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J. Pharm. Sci. 86, 1105–1110 (1997).
Krug, S. M. et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 20, 3713–3724 (2009).
Shen, L. et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 119, 2095–2106 (2006).
Su, L. et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136, 551–563 (2009).
Jou, T. S., Schneeberger, E. E. & Nelson, W. J. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 142, 101–115 (1998).
Schlegel, N., Meir, M., Spindler, V., Germer, C. T. & Waschke, J. Differential role of Rho GTPases in intestinal epithelial barrier regulation in vitro. J. Cell. Physiol. 226, 1196–1203 (2010).
Bruewer, M., Hopkins, A. M., Hobert, M. E., Nusrat, A. & Madara, J. L. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am. J. Physiol. Cell Physiol. 287, C327–C335 (2004).
Rojas, R., Ruiz, W. G., Leung, S. M., Jou, T. S. & Apodaca, G. Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 12, 2257–2274 (2001).
Ivanov, A. I., Samarin, S. N., Bachar, M., Parkos, C. A. & Nusrat, A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 10, 36 (2009).
Collares-Buzato, C. B., Jepson, M. A., Simmons, N. L. & Hirst, B. H. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur. J. Cell Biol. 76, 85–92 (1998).
Chen, Y., Lu, Q., Schneeberger, E. E. & Goodenough, D. A. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol. Biol. Cell 11, 849–862 (2000).
Ciccocioppo, R. et al. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am. J. Clin. Pathol. 125, 502–511 (2006).
Resta-Lenert, S., Smitham, J. & Barrett, K. E. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G153–G162 (2005).
Tsukamoto, T. & Nigam, S. K. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am. J. Physiol. 276, F737–F750 (1999).
Findley, M. K. & Koval, M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life 61, 431–437 (2009).
Ivanov, A. I., Nusrat, A. & Parkos, C. A. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 15, 176–188 (2004).
Amasheh, M. et al. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J. Nutr. 138, 1067–1073 (2008).
Yu, T. X. et al. Chk2-dependent HuR phosphorylation regulates occludin mRNA translation and epithelial barrier function. Nucleic Acids Res. 39, 8472–8487 (2011).
Youakim, A. & Ahdieh, M. Interferon-γ decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am. J. Physiol. 276, G1279–G1288 (1999).
Keita, A. V. & Soderholm, J. D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 22, 718–733 (2010).
Israel, E. J. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr. Suppl. 396, 27–32 (1994).
da Silva, M. E. et al. Diabetes mellitus-related autoantibodies in childhood autoimmune hepatitis. J. Pediatr. Endocrinol. Metab. 15, 831–840 (2002).
Gebbers, J. O. & Laissue, J. A. Bacterial translocation in the normal human appendix parallels the development of the local immune system. Ann. NY Acad. Sci. 1029, 337–343 (2004).
Tlaskalova-Hogenova, H. et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 8, 110–120 (2011).
De Quelen, F. et al. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J. Physiol. 589, 4341–4352 (2011).
Vaarala, O. Leaking gut in type 1 diabetes. Curr. Opin. Gastroenterol. 24, 701–706 (2008).
Weber, P., Brune, T., Ganser, G. & Zimmer, K. P. Gastrointestinal symptoms and permeability in patients with juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 21, 657–662 (2003).
de Magistris, L. et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 51, 418–424 (2010).
Soderholm, J. D. et al. Epithelial permeability to proteins in the noninflamed ileum of Crohn's disease? Gastroenterology 117, 65–72 (1999).
Soderholm, J. D. et al. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn's disease. Gut 50, 307–313 (2002).
Buhner, S. et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut 55, 342–347 (2006).
Arnott, I. D., Kingstone, K. & Ghosh, S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand. J. Gastroenterol. 35, 1163–1169 (2000).
D'Inca, R. et al. Intestinal permeability test as a predictor of clinical course in Crohn's disease. Am. J. Gastroenterol. 94, 2956–2960 (1999).
Wyatt, J., Vogelsang, H., Hubl, W., Waldhoer, T. & Lochs, H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341, 1437–1439 (1993).
Buning, C. et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm. Bowel Dis. 18, 1932–1939 (2012).
Schurmann, G., Bruwer, M. & Senninger, N. Ulcerative colitis: fate of pediatric ileoanal pouches [German]. Z. Gastroenterol. 37, 987–989 (1999).
Wallon, C., Braaf, Y., Wolving, M., Olaison, G. & Soderholm, J. D. Endoscopic biopsies in Ussing chambers evaluated for studies of macromolecular permeability in the human colon. Scand. J. Gastroenterol. 40, 586–595 (2005).
Olson, T. S. et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J. Exp. Med. 203, 541–552 (2006).
Reuter, B. K. & Pizarro, T. T. Mechanisms of tight junction dysregulation in the SAMP1/YitFc model of Crohn's disease-like ileitis. Ann. NY Acad. Sci. 1165, 301–307 (2009).
Madsen, K. L. et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 5, 262–270 (1999).
Arrieta, M. C., Madsen, K., Doyle, J. & Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 58, 41–48 (2009).
Piche, T. et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58, 196–201 (2009).
Ait-Belgnaoui, A., Bradesi, S., Fioramonti, J., Theodorou, V. & Bueno, L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 113, 141–147 (2005).
Dai, C., Zhao, D. H. & Jiang, M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int. J. Mol. Med. 29, 202–208 (2012).
Roka, R. et al. Colonic luminal proteases activate colonocyte proteinase-activated receptor-2 and regulate paracellular permeability in mice. Neurogastroenterol. Motil. 19, 57–65 (2007).
Okamoto, R. & Watanabe, M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig. Dis. Sci. 50 (Suppl. 1), S34–S38 (2005).
Fiorino, G., Cesarini, M., Indriolo, A. & Malesci, A. Mucosal healing in ulcerative colitis: where do we stand? Curr. Drug Targets 12, 1417–1423 (2011).
Flynn, A. & Kane, S. Mucosal healing in Crohn's disease and ulcerative colitis: what does it tell us? Curr. Opin. Gastroenterol. 27, 342–345 (2011).
Michetti, P. Assessment and importance of mucosal healing. Presented at UEGW 2011.
Gilbert, S. et al. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol. Med. 4, 109–124 (2012).
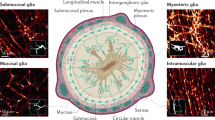
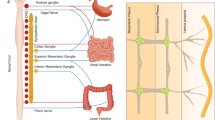
Wedel, T. et al. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann. Anat. 181, 327–337 (1999).
Grundy, D. & Schemann, M. Enteric nervous system. Curr. Opin. Gastroenterol. 22, 102–110 (2006).
Furness, J. B. et al. Sensitization of enteric reflexes in the rat colon in vitro. Auton. Neurosci. 97, 19–25 (2002).
Dogiel. Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere [German]. Arch. Anat. Physiol. Leipzig. Anat. Abt. Jg 130–158 (1899).
Gulbransen, B. D. & Sharkey, K. A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. http://dx.doi.org/10.1038/nrgastro.2012.138.
Cook, R. D. & Burnstock, G. The ultrastructure of Auerbach's plexus in the guinea-pig. II. Non-neuronal elements. J. Neurocytol. 5, 195–206 (1976).
Gabella, G. Glial cells in the myenteric plexus. Z. Naturforsch. B 26, 244–245 (1971).
Hanani, M. & Reichenbach, A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 278, 153–160 (1994).
Jessen, K. R. & Mirsky, R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J. Neurosci. 3, 2206–2218 (1983).
Ferri, G. L. et al. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 297, 409–410 (1982).
Hoff, S. et al. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 509, 356–371 (2008).
Sasselli, V., Pachnis, V. & Burns, A. J. The enteric nervous system. Dev. Biol. 366, 64–73 (2012).
Boesmans, W., van den Berghe, P. & Pachnis, V. Glial heterogeneity in the enteric nervous system [Abstract]. Neurogastroenterol. Motil. 24 (Suppl. s2), 36 (2012).
Song, Z. M., Brookes, S. J., Llewellyn-Smith, I. J. & Costa, M. Ultrastructural studies of the myenteric plexus and smooth muscle in organotypic cultures of the guinea-pig small intestine. Cell Tissue Res. 280, 627–637 (1995).
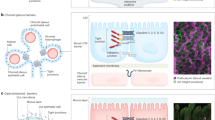
Neunlist, M. et al. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G1028–G1036 (2003).
Song, Z. M., Brookes, S. J., Steele, P. A. & Costa, M. Projections and pathways of submucous neurons to the mucosa of the guinea-pig small intestine. Cell Tissue Res. 269, 87–98 (1992).
Neunlist, M., Frieling, T., Rupprecht, C. & Schemann, M. Polarized enteric submucosal circuits involved in secretory responses of the guinea-pig proximal colon. J. Physiol. 506 (Pt 2), 539–550 (1998).
Neunlist, M. et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut 52, 84–90 (2003).
Porter, A. J., Wattchow, D. A., Brookes, S. J. & Costa, M. Projections of nitric oxide synthase and vasoactive intestinal polypeptide-reactive submucosal neurons in the human colon. J. Gastroenterol. Hepatol. 14, 1180–1187 (1999).
Mestres, P., Diener, M. & Rummel, W. Electron microscopy of the mucosal plexus of the rat colon. Acta Anat. (Basel) 143, 275–282 (1992).
Neunlist, M. et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G231–G241 (2007).
Van Landeghem, L. et al. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G976–G987 (2011).
Savidge, T. C., Sofroniew, M. V. & Neunlist, M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab. Invest. 87, 731–736 (2007).
Schemann, M. & Grundy, D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am. J. Physiol. 263, G709–G718 (1992).
Luyer, M. D. et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 202, 1023–1029 (2005).
Ghia, J. E., Blennerhassett, P., Kumar-Ondiveeran, H., Verdu, E. F. & Collins, S. M. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130 (2006).
Costantini, T. W. et al. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1308–G1318 (2010).
Meurette, G. et al. Sacral nerve stimulation enhances epithelial barrier of the rectum: results from a porcine model. Neurogastroenterol. Motil. 24, 267–273 (2012).
Greenwood, B. & Mantle, M. Mucin and protein release in the rabbit jejunum: effects of bethanechol and vagal nerve stimulation. Gastroenterology 103, 496–505 (1992).
van der Zanden, E. P. et al. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor α4β2. Gastroenterology 137, 1029–1039 (2009).
Boudry, G., Morise, A., Seve, B. & LE Huërou-Luron, I. Effect of milk formula protein content on intestinal barrier function in a porcine model of LBW neonates. Pediatr. Res. 69, 4–9 (2011).
Cameron, H. L. & Perdue, M. H. Muscarinic acetylcholine receptor activation increases transcellular transport of macromolecules across mouse and human intestinal epithelium in vitro. Neurogastroenterol. Motil. 19, 47–56 (2007).
Gareau, M. G., Jury, J. & Perdue, M. H. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G198–G203 (2007).
Sun, Y., Fihn, B. M., Sjovall, H. & Jodal, M. Enteric neurons modulate the colonic permeability response to luminal bile acids in rat colon in vivo. Gut 53, 362–367 (2004).
Wallon, C. et al. Eosinophils express muscarinic receptors and corticotropin-releasing factor to disrupt the mucosal barrier in ulcerative colitis. Gastroenterology 140, 1597–1607 (2011).
Hallgren, A., Flemstrom, G. & Nylander, O. Interaction between neurokinin A, VIP, prostanoids, and enteric nerves in regulation of duodenal function. Am. J. Physiol. 275, G95–G103 (1998).
Zakko, S., Barton, G., Weber, E., Dunger-Baldauf, C. & Ruhl, A. Randomised clinical trial: the clinical effects of a novel neurokinin receptor antagonist, DNK333, in women with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 33, 1311–1321 (2011).
Blais, A., Aymard, P. & Lacour, B. Paracellular calcium transport across Caco-2 and HT29 cell monolayers. Pflugers Arch. 434, 300–305 (1997).
Nylander, O. & Sjoblom, M. Modulation of mucosal permeability by vasoactive intestinal peptide or lidocaine affects the adjustment of luminal hypotonicity in rat duodenum. Acta Physiol. (Oxf.) 189, 325–335 (2007).
Conlin, V. S. et al. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G735–G750 (2009).
Ben-Horin, S. & Chowers, Y. Neuroimmunology of the gut: physiology, pathology, and pharmacology. Curr. Opin. Pharmacol. 8, 490–495 (2008).
Strassheim, D. et al. M3 muscarinic acetylcholine receptors regulate cytoplasmic myosin by a process involving RhoA and requiring conventional protein kinase C isoforms. J. Biol. Chem. 274, 18675–18685 (1999).
Bjerknes, M. & Cheng, H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc. Natl Acad. Sci. USA 98, 12497–12502 (2001).
Toumi, F. et al. Human submucosal neurones regulate intestinal epithelial cell proliferation: evidence from a novel co-culture model. Neurogastroenterol. Motil. 15, 239–242 (2003).
Cheng, K. et al. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G591–G597 (2008).
Goode, T. et al. Neurokinin-1 receptor (NK-1R) expression is induced in human colonic epithelial cells by proinflammatory cytokines and mediates proliferation in response to substance P. J. Cell. Physiol. 197, 30–41 (2003).
Gross, E. R., Gershon, M. D., Margolis, K. G., Gertsberg, Z. V. & Cowles, R. A. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143, 408–417 e402 (2012).
Wright, K. et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology 129, 437–453 (2005).
Konturek, P. C. et al. Role of brain-gut axis in healing of gastric ulcers. J. Physiol. Pharmacol. 55, 179–192 (2004).
Bulut, K. et al. Sensory neuropeptides and epithelial cell restitution: the relevance of SP- and CGRP-stimulated mast cells. Int. J. Colorectal Dis. 23, 535–541 (2008).
Felderbauer, P. et al. Substance P induces intestinal wound healing via fibroblasts—evidence for a TGF-β-dependent effect. Int. J. Colorectal Dis. 22, 1475–1480 (2007).
Aube, A. C. et al. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 55, 630–637 (2006).
Bush, T. G. et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201 (1998).
Cornet, A. et al. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc. Natl Acad. Sci. USA 98, 13306–13311 (2001).
Savidge, T. C. et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132, 1344–1358 (2007).
Nasser, Y. et al. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G912–G927 (2006).
Flamant, M. et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut 60, 473–484 (2011).
Dijkstra, G., van Goor, H., Jansen, P. L. & Moshage, G. Targeting nitric oxide in the gastrointestinal tract. Curr. Opin. Investig. Drugs 5, 529–536 (2004).
Xiao, W. D. et al. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol. Cell. Neurosci. 46, 527–534 (2011).
MacEachern, S. J., Patel, B. A., McKay, D. M. & Sharkey, K. A. Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J. Physiol. 589, 3333–3348 (2011).
Steinkamp, M. et al. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 124, 1748–1757 (2003).
Zhang, D. K. et al. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J. Pathol. 222, 213–222 (2010).
Anitha, M. et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Invest. 116, 344–356 (2006).
Baudry, C. et al. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J. Physiol. 590, 533–544 (2011).
Costantini, T. W. et al. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am. J. Pathol. 181, 478–486 (2012).
Bach-Ngohou, K. et al. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2. J. Physiol. 588, 2533–2544 (2010).
Van Landeghem, L. et al. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics 10, 507 (2009).
Esposito, G. et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 133, 918–925 (2007).
Wedel, T., Krammer, H. J., Kuhnel, W. & Sigge, W. Alterations of the enteric nervous system in neonatal necrotizing enterocolitis revealed by whole-mount immunohistochemistry. Pediatr. Pathol. Lab. Med. 18, 57–70 (1998).
Rolli-Derkinderen, M. et al. Enteric glial cells from patients with Crohn's disease misreact to inflammation and induce intestinal epithelial cell permeability [Abstract]. Neurogastroenterol. Motil. 24 (Suppl. s2), 44 (2012).
von Boyen, G. B. et al. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 11, 3 (2011).
Hamby, M. E. & Sofroniew, M. V. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics 7, 494–506 (2010).
Cirillo, C. et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 21, 1209-e1112 (2009).
Cirillo, C. et al. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol. Motil. 23, e372–w382 (2011).
Villanacci, V. et al. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol. Motil. 20, 1009–1016 (2008).
Ruhl, A., Franzke, S., Collins, S. M. & Stremmel, W. Interleukin-6 expression and regulation in rat enteric glial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G1163–G1171 (2001).
Bradley, J. S. Jr, Parr, E. J. & Sharkey, K. A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 289, 455–461 (1997).
von Boyen, G. B. et al. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53, 222–228 (2004).
Kirchgessner, A. L., Liu, M. T., Raymond, J. R. & Gershon, M. D. Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J. Comp. Neurol. 364, 439–455 (1996).
Cooke, H. J., Sidhu, M. & Wang, Y. Z. 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol. Motil. 9, 181–186 (1997).
Crone, S. A., Negro, A., Trumpp, A., Giovannini, M. & Lee, K. F. Colonic epithelial expression of ErbB2 is required for postnatal maintenance of the enteric nervous system. Neuron 37, 29–40 (2003).
Moriez, R. et al. Neuroplasticity and neuroprotection in enteric neurons: role of epithelial cells. Biochem. Biophy. Res. Commun. 382, 577–582 (2009).
Tixier, E., Galmiche, J. P. & Neunlist, M. Intestinal neuro-epithelial interactions modulate neuronal chemokines production. Bioche. Biophys. Res. Commun. 344, 554–561 (2006).
Tixier, E., Lalanne, F., Just, I., Galmiche, J. P. & Neunlist, M. Human mucosa/submucosa interactions during intestinal inflammation: involvement of the enteric nervous system in interleukin-8 secretion. Cell. Microbiol. 7, 1798–1810 (2005).
Bertrand, P. P., Kunze, W. A., Bornstein, J. C., Furness, J. B. & Smith, M. L. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol. 273, G422–G435 (1997).
Grider, J. R. & Piland, B. E. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G429–G437 (2007).
Soret, R. et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138, 1772–1782 (2010).
van Haver, E. R. et al. Postnatal and diet-dependent increases in enteric glial cells and VIP-containing neurones in preterm pigs. Neurogastroenterol. Motil. 20, 1070–1079 (2008).
Lebouvier, T. et al. Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol. Motil. 22, e11–14 (2010).
Cirillo, C., Tack, J. & Vanden Berghe, P. Nerve activity recordings in routine human intestinal biopsies. Gut http://dx.doi.org/gutjnl-2011-301777.
Buhner, S. et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137, 1425–1434 (2009).
Cenac, N. et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 117, 636–647 (2007).
Acknowledgements
Work in our laboratory is financed by grants from the INSERM, University of Nantes, Fondation pour la Recherche Médicale, INCa, ANR-EnteNeurObesity, INSERM-DHOS, Ligue contre le Cancer, Fondation SanTDige, France Parkinson, Michael J. Fox Fondation, CECAP (Comité d'Entente et de Coordination des Associations de Parkinsoniens) and Parkinsoniens de Vendée.
Author information
Authors and Affiliations
Contributions
M. Neunlist and L. Van Landeghem contributed to all aspects of this manuscript. M. Mahé contributed to researching data, writing and reviewing/editing the manuscript. P. Derkinderen contributed to discussion of content and reviewing/editing the manuscript. M. Rolli-Derkinderen contributed to discussion of content, writing and reviewing/editing the manuscript. S. Bruley des Varannes contributed to reviewing/editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Neunlist, M., Van Landeghem, L., Mahé, M. et al. The digestive neuronal–glial–epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10, 90–100 (2013). https://doi.org/10.1038/nrgastro.2012.221
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.221
This article is cited by
-
Dietary supplementation of benzoic acid and essential oils combination enhances intestinal resilience against LPS stimulation in weaned piglets
Journal of Animal Science and Biotechnology (2024)
-
The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases
Science China Life Sciences (2024)
-
Enteric glial cells aggravate the intestinal epithelial barrier damage by secreting S100β under high-altitude conditions
Molecular Biomedicine (2023)
-
Visualizing enteric nervous system activity through dye-free dynamic full-field optical coherence tomography
Communications Biology (2023)
-
Neuroimmune Connectomes in the Gut and Their Implications in Parkinson’s Disease
Molecular Neurobiology (2023)