Key Points
-
Patients with type 1 diabetes mellitus or type 2 diabetes mellitus (T2DM) have an increased risk of fractures; BMD underestimates this risk in individuals with T2DM, making risk assessment challenging
-
Patients with diabetes mellitus with long-term disease, poor glycaemic control, β-cell failure and who receive insulin treatment are at the highest risk of fractures
-
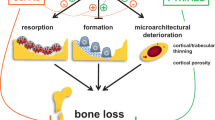
Low bone turnover, accumulation of advanced glycation endproducts, micro and macro-architecture alterations and tissue material damage lead to abnormal biomechanical properties and impair bone strength
-
Other determinants of bone fragility include inflammation, oxidative stress, adipokine alterations, WNT dysregulation and increased marrow fat
-
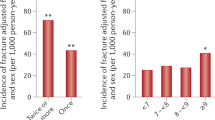
Complications of diabetes mellitus, such as neuropathy, poor balance, sarcopenia, vision impairment and frequent hypoglycaemic events, increase the risk of falls and risk of fracture; preventive measures are advised, especially in patients taking insulin
-
Use of thiazolidinediones, or some SGLT2 inhibitors might contribute to increased fracture risk; antidiabetic medications with good bone safety profiles such as metformin, GLP1analogues or DPP4 inhibitors are preferred
Abstract
The risk of fragility fractures is increased in patients with either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM). Although BMD is decreased in T1DM, BMD in T2DM is often normal or even slightly elevated compared with an age-matched control population. However, in both T1DM and T2DM, bone turnover is decreased and the bone material properties and microstructure of bone are altered; the latter particularly so when microvascular complications are present. The pathophysiological mechanisms underlying bone fragility in diabetes mellitus are complex, and include hyperglycaemia, oxidative stress and the accumulation of advanced glycation endproducts that compromise collagen properties, increase marrow adiposity, release inflammatory factors and adipokines from visceral fat, and potentially alter the function of osteocytes. Additional factors including treatment-induced hypoglycaemia, certain antidiabetic medications with a direct effect on bone and mineral metabolism (such as thiazolidinediones), as well as an increased propensity for falls, all contribute to the increased fracture risk in patients with diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas 6th edn (International Diabetes Federation, 2013).
Johnell, O. & Kanis, J. A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733 (2006).
Melton, L. J. 3rd, Chrischilles, E. A., Cooper, C., Lane, A. W. & Riggs, B. L. Perspective. How many women have osteoporosis? J. Bone Miner. Res. 7, 1005–1010 (1992).
Sanchez-Riera, L. et al. The global burden attributable to low bone mineral density. Ann. Rheum. Dis. 73, 1635–1645 (2014).
Janghorbani, M., Van Dam, R. M., Willett, W. C. & Hu, F. B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 166, 495–505 (2007).
Janghorbani, M., Feskanich, D., Willett, W. C. & Hu, F. Prospective study of diabetes and risk of hip fracture: the Nurses' Health Study. Diabetes Care 29, 1573–1578 (2006).
Weber, D. R., Haynes, K., Leonard, M. B., Willi, S. M. & Denburg, M. R. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using the Health Improvement Network (THIN). Diabetes Care 38, 1913–1920 (2015).
Vestergaard, P., Rejnmark, L. & Mosekilde, L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 48, 1292–1299 (2005).
Kelsey, J. L., Browner, W. S., Seeley, D. G., Nevitt, M. C. & Cummings, S. R. Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am. J. Epidemiol. 135, 477–489 (1992).
Zhukouskaya, V. V. et al. Prevalence of morphometric vertebral fractures in patients with type 1 diabetes. Diabetes Care 36, 1635–1640 (2013).
Ivers, R. Q., Cumming, R. G., Mitchell, P. & Peduto, A. J. Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care 24, 1198–1203 (2001).
Miao, J., Brismar, K., Nyren, O., Ugarph-Morawski, A. & Ye, W. Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden. Diabetes Care 28, 2850–2855 (2005).
Strotmeyer, E. S. et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch. Intern. Med. 165, 1612–1617 (2005).
Schwartz, A. V. et al. Older women with diabetes have an increased risk of fracture: a prospective study. J. Clin. Endocrinol. Metab. 86, 32–38 (2001).
Forsen, L., Meyer, H. E., Midthjell, K. & Edna, T. H. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag Health Survey. Diabetologia 42, 920–925 (1999).
Ahmed, L. A., Joakimsen, R. M., Berntsen, G. K., Fonnebo, V. & Schirmer, H. Diabetes mellitus and the risk of non-vertebral fractures: the Tromso study. Osteoporos. Int. 17, 495–500 (2006).
Napoli, N. et al. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia 57, 2057–2065 (2014).
Li, C. I. et al. Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan Diabetes Cohort Study. J. Bone Miner. Res. 30, 1338–1346 (2015).
Johnston, S. S., Conner, C., Aagren, M., Ruiz, K. & Bouchard, J. Association between hypoglycaemic events and fall-related fractures in Medicare-covered patients with type 2 diabetes. Diabetes Obes. Metab. 14, 634–643 (2012).
Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes — a meta-analysis. Osteoporos. Int. 18, 427–444 (2007).
Napoli, N. et al. Risk factors for subtrochanteric and diaphyseal fractures: the study of osteoporotic fractures. J. Clin. Endocrinol. Metab. 98, 659–667 (2013).
de Liefde, I. I. et al. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos. Int. 16, 1713–1720 (2005).
Bonds, D. E. et al. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 91, 3404–3410 (2006).
Yamamoto, M., Yamaguchi, T., Yamauchi, M., Kaji, H. & Sugimoto, T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J. Bone Miner. Res. 24, 702–709 (2009).
Lipscombe, L. L., Jamal, S. A., Booth, G. L. & Hawker, G. A. The risk of hip fractures in older individuals with diabetes: a population-based study. Diabetes Care 30, 835–841 (2007).
Schwartz, A. V. et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 305, 2184–2192 (2011).
Dubey, A., Aharonoff, G. B., Zuckerman, J. D. & Koval, K. J. The effects of diabetes on outcome after hip fracture. Bull. Hosp. Jt Dis. 59, 94–98 (2000).
Huang, Y. F. et al. Diabetes and health outcomes among older Taiwanese with hip fracture. Rejuvenation Res. 15, 476–482 (2012).
Muraki, S., Yamamoto, S., Ishibashi, H. & Nakamura, K. Factors associated with mortality following hip fracture in Japan. J. Bone Miner. Metab. 24, 100–104 (2006).
Schwartz, A. V. et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 25, 1749–1754 (2002).
Maurer, M. S., Burcham, J. & Cheng, H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1157–1162 (2005).
Gregg, E. W., Pereira, M. A. & Caspersen, C. J. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J. Am. Geriatr. Soc. 48, 883–893 (2000).
Schwartz, A. V. et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care 31, 391–396 (2008).
Hewston, P. & Deshpande, N. Falls and balance impairments in older adults with type 2 diabetes: thinking beyond diabetic peripheral neuropathy. Can. J. Diabetes 40, 6–9 (2016).
Berlie, H. D. & Garwood, C. L. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann. Pharmacother. 44, 712–717 (2010).
Vinik, A. I., Vinik, E. J., Colberg, S. R. & Morrison, S. Falls risk in older adults with type 2 diabetes. Clin. Geriatr. Med. 31, 89–99 (2015).
Campos Pastor, M. M., Lopez-Ibarra, P. J., Escobar-Jimenez, F., Serrano Pardo, M. D. & Garcia-Cervigon, A. G. Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: a prospective study. Osteoporos. Int. 11, 455–459 (2000).
Eller-Vainicher, C. et al. Low bone mineral density and its predictors in type 1 diabetic patients evaluated by the classic statistics and artificial neural network analysis. Diabetes Care 34, 2186–2191 (2011).
Joshi, A., Varthakavi, P., Chadha, M. & Bhagwat, N. A study of bone mineral density and its determinants in type 1 diabetes mellitus. J. Osteoporos. 2013, 397814 (2013).
Soto, N. et al. Bone mass and sex steroids in postmenarcheal adolescents and adult women with type 1 diabetes mellitus. J. Diabetes Comp. 25, 19–24 (2011).
Hampson, G. et al. Bone mineral density, collagen type 1 α 1 genotypes and bone turnover in premenopausal women with diabetes mellitus. Diabetologia 41, 1314–1320 (1998).
Mastrandrea, L. D. et al. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care 31, 1729–1735 (2008).
Lopez-Ibarra, P. J. et al. Bone mineral density at time of clinical diagnosis of adult-onset type 1 diabetes mellitus. Endocr. Pract. 7, 346–351 (2001).
Ma, L. et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur. J. Epidemiol. 27, 319–332 (2012).
Weinfeld, R. M., Olson, P. N., Maki, D. D. & Griffiths, H. J. The prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in two large American Midwest metropolitan hospital populations. Skeletal Radiol. 26, 222–225 (1997).
Dhaliwal, R., Cibula, D., Ghosh, C., Weinstock, R. S. & Moses, A. M. Bone quality assessment in type 2 diabetes mellitus. Osteoporos. Int. 25, 1969–1973 (2014).
Leslie, W. D., Aubry-Rozier, B., Lamy, O., Hans, D. & Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J. Clin. Endocrinol. Metab. 98, 602–609 (2013).
Rubin, J. et al. Trabecular bone assessment in type 2 diabetes mellitus. J. Bone Miner. Res. 28, S398 (2013).
Tao, B. et al. Differences between measurements of bone mineral densities by quantitative ultrasound and dual-energy X-ray absorptiometry in type 2 diabetic postmenopausal women. J. Clin. Endocrinol. Metab. 93, 1670–1675 (2008).
Yamaguchi, T. et al. Quantitative ultrasound and vertebral fractures in patients with type 2 diabetes. J. Bone Miner. Metab. 29, 626–632 (2011).
Bechtold, S. et al. Early manifestation of type 1 diabetes in children is a risk factor for changed bone geometry: data using peripheral quantitative computed tomography. Pediatrics 118, e627–e634 (2006).
Danielson, K. K., Elliott, M. E., LeCaire, T., Binkley, N. & Palta, M. Poor glycemic control is associated with low BMD detected in premenopausal women with type 1 diabetes. Osteoporos. Int. 20, 923–933 (2009).
Forst, T. et al. Peripheral osteopenia in adult patients with insulin-dependent diabetes mellitus. Diabet. Med. 12, 874–879 (1995).
Heap, J., Murray, M. A., Miller, S. C., Jalili, T. & Moyer-Mileur, L. J. Alterations in bone characteristics associated with glycemic control in adolescents with type 1 diabetes mellitus. J. Pediatr. 144, 56–62 (2004).
Lettgen, B., Hauffa, B., Mohlmann, C., Jeken, C. & Reiners, C. Bone mineral density in children and adolescents with juvenile diabetes: selective measurement of bone mineral density of trabecular and cortical bone using peripheral quantitative computed tomography. Hormone Res. 43, 173–175 (1995).
Saha, M. T., Sievanen, H., Salo, M. K., Tulokas, S. & Saha, H. H. Bone mass and structure in adolescents with type 1 diabetes compared to healthy peers. Osteoporos. Ont. 20, 1401–1406 (2009).
Roggen, I., Gies, I., Vanbesien, J., Louis, O. & De Schepper, J. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Hormone Res. Paediatr. 79, 68–74 (2013).
Pritchard, J. M. et al. Changes in trabecular bone microarchitecture in postmenopausal women with and without type 2 diabetes: a two year longitudinal study. BMC Musculoskelet. Disord. 14, 114 (2013).
Pritchard, J. M. et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res. 64, 83–91 (2012).
Burghardt, A. J. et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 95, 5045–5055 (2010).
Farr, J. N. et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J. Bone Miner. Res. 29, 787–795 (2014).
Patsch, J. M. et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J. Bone Miner. Res. 28, 313–324 (2013).
Shanbhogue, V. V. et al. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur. J. Endocrinol. 174, 115–124 (2016).
Bala, Y. et al. Trabecular and cortical microstructure and fragility of the distal radius in women. J. Bone Miner. Res. 30, 621–629 (2015).
Yu, E. W. et al. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos. Int. 26, 673–679 (2015).
Ogawa, N. et al. The combination of high glucose and advanced glycation end-products (AGEs) inhibits the mineralization of osteoblastic MC3T3-E1 cells through glucose-induced increase in the receptor for AGEs. Horm. Metab. Res. 39, 871–875 (2007).
Hough, F. S. et al. Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur. J. Endocrinol. 174, R127–138 (2016).
Napoli, N. et al. The alliance of mesenchymal stem cells, bone, and diabetes. Int. J. Endocrinol. 2014, 690783 (2014).
Maggio, A. B. et al. Decreased bone turnover in children and adolescents with well controlled type 1 diabetes. J. Pediatr. Endocrinol. Metab. 23, 697–707 (2010).
Adami, S. Bone health in diabetes: considerations for clinical management. Curr. Med. Res. Opin. 25, 1057–1072 (2009).
Diaz-Lopez, A. et al. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study. J. Clin. Endocrinol. Metab. 98, 4524–4531 (2013).
Kanazawa, I. et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 94, 45–49 (2009).
Starup-Linde, J., Eriksen, S. A., Lykkeboe, S., Handberg, A. & Vestergaard, P. Biochemical markers of bone turnover in diabetes patients — a meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporos. Int. 25, 1697–1708 (2014).
Starup-Linde, J. & Vestergaard, P. Biochemical bone turnover markers in diabetes mellitus — a systematic review. Bone 82, 69–78 (2016).
Leite Duarte, M. E. & da Silva, R. D. [Histomorphometric analysis of the bone tissue in patients with non-insulin-dependent diabetes (DMNID)]. Rev. Hosp. Clin. Fac. Med. Sao Paulo 51, 7–11 (in Portuguese) (1996).
Manavalan, J. S. et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 97, 3240–3250 (2012).
Armas, L. A., Akhter, M. P., Drincic, A. & Recker, R. R. Trabecular bone histomorphometry in humans with type 1 diabetes mellitus. Bone 50, 91–96 (2012).
Farlay, D. et al. Nonenzymatic glycation and degree of mineralization are higher in bone from fractured patients with type 1 diabetes mellitus. J. Bone Miner. Res. 31, 190–195 (2016).
Reyes-Garcia, R. et al. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 50, 47–52 (2013).
Yamamoto, M., Yamaguchi, T., Nawata, K., Yamauchi, M. & Sugimoto, T. Decreased PTH levels accompanied by low bone formation are associated with vertebral fractures in postmenopausal women with type 2 diabetes. J. Clin. Endocrinol. Metab. 97, 1277–1284 (2012).
McNair, P., Christensen, M. S., Madsbad, S., Christiansen, C. & Transbol, I. Hypoparathyroidism in diabetes mellitus. Acta Endocrinol. 96, 81–86 (1981).
Thalassinos, N. C., Hadjiyanni, P., Tzanela, M., Alevizaki, C. & Philokiprou, D. Calcium metabolism in diabetes mellitus: effect of improved blood glucose control. Diabet. Med. 10, 341–344 (1993).
Weyer, C. et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 86, 1930–1935 (2001).
Williams, G. A. et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology 150, 3603–3610 (2009).
Lenchik, L. et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33, 646–651 (2003).
Napoli, N. et al. Adiponectin and bone mass density: The InCHIANTI study. Bone 47, 1001–1005 (2010).
Tamura, T. et al. Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism 56, 623–628 (2007).
Hamrick, M. W. & Ferrari, S. L. Leptin and the sympathetic connection of fat to bone. Osteoporos. Int. 19, 905–912 (2008).
Nuche-Berenguer, B. et al. Exendin-4 exerts osteogenic actions in insulin-resistant and type 2 diabetic states. Regul. Pept. 159, 61–66 (2010).
Gennari, L. et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J. Clin. Endocrinol. Metab. 97, 1737–1744 (2012).
Gaudio, A. et al. Sclero stin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 97, 3744–3750 (2012).
Yamamoto, M., Yamauchi, M. & Sugimoto, T. Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 98, 4030–4037 (2013).
Heilmeier, U. et al. Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos. Int. 26, 1283–1293 (2015).
Bucala, R. & Vlassara, H. Advanced glycosylation end products in diabetic renal and vascular disease. Am. J. Kidney Dis. 26, 875–888 (1995).
Hein, G. E. Glycation endproducts in osteoporosis — is there a pathophysiologic importance? Clin. Chim. Acta 371, 32–36 (2006).
Leslie, W. D., Rubin, M. R., Schwartz, A. V. & Kanis, J. A. Type 2 diabetes and bone. J. Bone Miner. Res. 27, 2231–2237 (2012).
Vashishth, D. et al. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28, 195–201 (2001).
Saito, M., Fujii, K., Mori, Y. & Marumo, K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos. Int. 17, 1514–1523 (2006).
Kerkeni, M., Saidi, A., Bouzidi, H., Ben Yahya, S. & Hammami, M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc. Res. 84, 378–383 (2012).
Saito, M., Fujii, K. & Marumo, K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif. Tissue Int. 79, 160–168 (2006).
Schwartz, A. V. et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J. Clin. Endocrinol. Metab. 94, 2380–2386 (2009).
Yamamoto, M., Yamaguchi, T., Yamauchi, M., Yano, S. & Sugimoto, T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J. Clin. Endocrinol. Metab. 93, 1013–1019 (2008).
Takagi, M. et al. Advanced glycation endproducts stimulate interleukin-6 production by human bone-derived cells. J. Bone Miner. Res. 12, 439–446 (1997).
Yamamoto, T. et al. Role of advanced glycation end products in adynamic bone disease in patients with diabetic nephropathy. Am. J. Kidney Dis. 38, S161–S164 (2001).
Katayama, Y., Akatsu, T., Yamamoto, M., Kugai, N. & Nagata, N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J. Bone Miner. Res. 11, 931–937 (1996).
Kume, S. et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J. Bone Miner. Res. 20, 1647–1658 (2005).
Sanguineti, R., Storace, D., Monacelli, F., Federici, A. & Odetti, P. Pentosidine effects on human osteoblasts in vitro. Ann. NY Acad. Sci. 1126, 166–172 (2008).
McCarthy, A. D., Uemura, T., Etcheverry, S. B. & Cortizo, A. M. Advanced glycation endproducts interefere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int. J. Biochem. Cell Biol. 36, 840–848 (2004).
Yonekura, H. et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 370, 1097–1109 (2003).
Yamamoto, M., Yamaguchi, T., Yamauchi, M. & Sugimoto, T. Low serum level of the endogenous secretory receptor for advanced glycation end products (esRAGE) is a risk factor for prevalent vertebral fractures independent of bone mineral density in patients with type 2 diabetes. Diabetes Care 32, 2263–2268 (2009).
Cunha, J. S., Ferreira, V. M., Maquigussa, E., Naves, M. A. & Boim, M. A. Effects of high glucose and high insulin concentrations on osteoblast function in vitro. Cell Tissue Res. 358, 249–256 (2014).
Zayzafoon, M., Stell, C., Irwin, R. & McCabe, L. R. Extracellular glucose influences osteoblast differentiation and c-Jun expression. J. Cell. Biochem. 79, 301–310 (2000).
Botolin, S. & McCabe, L. R. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J. Cell. Biochem. 99, 411–424 (2006).
Wei, J. et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J. Clin. Invest. 124, 1–13 (2014).
Balint, E., Szabo, P., Marshall, C. F. & Sprague, S. M. Glucose-induced inhibition of in vitro bone mineralization. Bone 28, 21–28 (2001).
Frassetto, L. A. & Sebastian, A. How metabolic acidosis and oxidative stress alone and interacting may increase the risk of fracture in diabetic subjects. Med. Hypotheses 79, 189–192 (2012).
Aguiari, P. et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc. Natl Acad. Sci. USA 105, 1226–1231 (2008).
Oei, L. et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care 36, 1619–1628 (2013).
Yang, J., Zhang, X., Wang, W. & Liu, J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem. Funct. 28, 334–341 (2010).
Gandhi, A., Beam, H. A., O'Connor, J. P., Parsons, J. R. & Lin, S. S. The effects of local insulin delivery on diabetic fracture healing. Bone 37, 482–490 (2005).
Fulzele, K. et al. Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J. Biol. Chem. 282, 25649–25658 (2007).
McCarthy, A. D., Etcheverry, S. B. & Cortizo, A. M. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol. 38, 113–122 (2001).
Terada, M. et al. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone 22, 17–23 (1998).
Kanazawa, I., Yamaguchi, T. & Sugimoto, T. Serum insulin-like growth factor-I is a marker for assessing the severity of vertebral fractures in postmenopausal women with type 2 diabetes mellitus. Osteoporos. Int. 22, 1191–1198 (2011).
Cornish, J. & Naot, D. Amylin and adrenomedullin: novel regulators of bone growth. Curr. Pharm. Design 8, 2009–2021 (2002).
Gilbert, L. et al. Inhibition of osteoblast differentiation by tumor necrosis factor-α. Endocrinology 141, 3956–3964 (2000).
Glantschnig, H., Fisher, J. E., Wesolowski, G., Rodan, G. A. & Reszka, A. A. M-CSF TNFα and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 10, 1165–1177 (2003).
Manolagas, S. C. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31, 266–300 (2010).
Hotta, K. et al. Age-related adipose tissue mRNA expression of ADD1/SREBP1, PPARγ, lipoprotein lipase, and GLUT4 glucose transporter in rhesus monkeys. J. Gerontol. A Biol. Sci. Med. Sci. 54, B183–B188 (1999).
Dong, X. et al. FFAs–ROS–ERK/P38 pathway plays a key role in adipocyte lipotoxicity on osteoblasts in co-culture. Biochimie 101, 123–131 (2014).
Blonde, L. et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes. Metab. 8, 436–447 (2006).
Fazeli, P. K. et al. Marrow fat and bone — new perspectives. J. Clin. Endocrinol. Metab. 98, 935–945 (2013).
Giralt, M. & Villarroya, F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154, 2992–3000 (2013).
Rahman, S. et al. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 154, 2687–2701 (2013).
Singh, R., Braga, M. & Pervin, S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front. Cell Dev. Biol. 2, 60 (2014).
Knop, F. K. et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56, 1951–1959 (2007).
Nuche-Berenguer, B. et al. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J. Cell. Physiol. 225, 585–592 (2010).
Sanz, C. et al. Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am. J. Physiol. Endocrinol. Metab. 298, E634–E643 (2010).
Kim, J. Y. et al. Exendin-4 increases bone mineral density in type 2 diabetic OLETF rats potentially through the down-regulation of SOST/sclerostin in osteocytes. Life Sci. 92, 533–540 (2013).
Ma, X. et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J. Bone Miner. Res. 28, 1641–1652 (2013).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321, 405–412 (2000).
Schneider, A. L. et al. Diabetes and risk of fracture-related hospitalization: the Atherosclerosis Risk in Communities Study. Diabetes Care 36, 1153–1158 (2013).
Schwartz, A. V. et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care 35, 1525–1531 (2012).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38, 140–149 (2015).
Villareal, D. T. et al. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 364, 1218–1229 (2011).
Shah, K. et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J. Bone Miner. Res. 26, 2851–2859 (2011).
Armamento-Villareal, R. et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J. Bone Miner. Res. 27, 1215–1221 (2012).
Palermo, A. et al. Oral anti-diabetic drugs and fracture risk, cut to the bone: safe or dangerous? A narrative review. Osteoporos. Int. 26, 2073–2089 (2015).
Monami, M. et al. Bone fractures and hypoglycemic treatment in type 2 diabetic patients: a case-control study. Diabetes Care 31, 199–203 (2008).
Kanazawa, I., Yamaguchi, T., Yano, S., Yamauchi, M. & Sugimoto, T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem. Biophys. Res. Commun. 375, 414–419 (2008).
Solomon, D. H. et al. A cohort study of thiazolidinediones and fractures in older adults with diabetes. J. Clin. Endocrinol. Metab. 94, 2792–2798 (2009).
Del Prato, S., Camisasca, R., Wilson, C. & Fleck, P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes Obes. Metab. 16, 1239–1246 (2014).
Kawai, M. & Rosen, C. J. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 6, 629–636 (2010).
Loke, Y. K., Singh, S. & Furberg, C. D. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 180, 32–39 (2009).
Toulis, K. A., Goulis, D. G. & Anastasilakis, A. D. Thiazolidinedione use and the risk of fractures. CMAJ 180, 841–842; author reply 842–843 (2009).
Zhu, Z. N., Jiang, Y. F. & Ding, T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone 68, 115–123 (2014).
Mabilleau, G., Mieczkowska, A. & Chappard, D. Use of glucagon-like peptide-1 receptor agonists and bone fractures: a meta-analysis of randomized clinical trials. J. Diabetes 6, 260–266 (2014).
Su, B. et al. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists' treatment: a meta-analysis of randomized controlled trials. Endocrine 48, 107–115 (2015).
Monami, M., Dicembrini, I., Antenore, A. & Mannucci, E. Dipeptidyl peptidase-4 inhibitors and bone fractures: a meta-analysis of randomized clinical trials. Diabetes Care 34, 2474–2476 (2011).
Mosenzon, O. et al. Incidence of fractures in patients with type 2 diabetes in the SAVOR-TIMI 53 trial. Diabetes Care 38, 2142–2150 (2015).
DeFronzo, R. A., Davidson, J. A. & Del Prato, S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes. Metab. 14, 5–14 (2012).
Ljunggren, O. et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes. Metab. 14, 990–999 (2012).
Bilezikian, J. P. et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J. Clin. Endocrinol. Metab. 101, 44–51 (2016).
Watts, N. B. et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 101, 157–166 (2016).
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998).
U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 44, 1249–1258 (1995).
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352, 854–865 (1998).
Kahn, S. E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 (2006).
Lebovitz, H. E. Insulin secretagogues: old and new. Diabetes Rev. 7, 139–153 (1999).
Aguirre, L. et al. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J. Clin. Endocrinol. Metab. 99, 3290–3297 (2014).
Ma, Y. H. et al. Circulating sclerostin associated with vertebral bone marrow fat in older men but not women. J. Clin. Endocrinol. Metab. 99, E2584–E2590 (2014).
Napoli, N. et al. Effect of ghrelin on bone mass density: The InChianti study. Bone. 49, 257–263 (2011).
Iacobellis, G. et al. Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J. Endocrinol. Invest. 34, e12–e15 (2011).
Acknowledgements
The authors acknowledge the support of the other members of the CSA IOF Bone and Diabetes Working Group: K. Akesson, M. S. M. Ardawi, C. Cooper, R. Eastell, G. El Hajj Fuleihan, S. Hough, R. Josse, D. Kendler, M. Kraenzlin, W. Leslie, M. Massi Benedetti, A. Mithal and A. Suzuki.
Author information
Authors and Affiliations
Consortia
Contributions
N.N. and D.D.P. researched data for the article. N.N., M.C., B.A., A.V.S. and S.L.F. made substantial contributions to discussions of the content. N.N., M.C., B.A., A.V.S. and S.L.F. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.N. has served as a consultant for Amgen and Takeda. A.V.S is a consultant for Amgen and Jansen. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Napoli, N., Chandran, M., Pierroz, D. et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol 13, 208–219 (2017). https://doi.org/10.1038/nrendo.2016.153
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.153
This article is cited by
-
Biochanin A abrogates osteoclastogenesis in type 2 diabetic osteoporosis via regulating ROS/MAPK signaling pathway based on integrating molecular docking and experimental validation
BMC Complementary Medicine and Therapies (2024)
-
Identification of osteoporosis ferroptosis-related markers and potential therapeutic compounds based on bioinformatics methods and molecular docking technology
BMC Medical Genomics (2024)
-
Diabetes and osteoporosis: a two-sample mendelian randomization study
BMC Musculoskeletal Disorders (2024)
-
The impact of diabetes, anemia, and renal function in the relationship between osteoporosis and fasting blood glucose among Taiwanese women: a cross-sectional study
BMC Women's Health (2024)
-
Association of type 2 Diabetes Mellitus and bone mineral density: a two-sample Mendelian randomization study
BMC Musculoskeletal Disorders (2024)