Key Points
-
RET proto-oncogene alterations are crucial events for thyroid cancer development
-
Different mechanisms of RET activation, point mutations and gene rearrangements characterize medullary and papillary thyroid carcinoma
-
RET genetic screening is an important tool for the diagnosis of patients with medullary thyroid cancer and can assist in the presurgical diagnosis of papillary thyroid cancer
-
RET mutations are a very strong factor for poor prognosis in medullary thyroid cancer
-
RET/PTC rearrangements, especially RET/PTC3, are associated with the most aggressive histological variant of papillary thyroid cancer
-
The knowledge of tumour biology is crucial for the identification of drugs able to block tumour development
Abstract
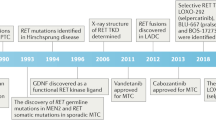
The rearranged during transfection (RET) proto-oncogene was identified in 1985 and, very soon thereafter, a rearrangement named RET/PTC was discovered in papillary thyroid carcinoma (PTC). After this discovery, other RET rearrangements were found in PTCs, particularly in those induced by radiation. For many years, it was thought that these genetic alterations only occurred in PTC, but, in the past couple of years, some RET/PTC rearrangements have been found in other human tumours. 5 years after the discovery of RET/PTC rearrangements in PTC, activating point mutations in the RET proto-oncogene were discovered in both hereditary and sporadic forms of medullary thyroid carcinoma (MTC). In contrast to the alterations found in PTC, the activation of RET in MTC is mainly due to activating point mutations. Interestingly, in the past year, RET rearrangements that were different to those described in PTC were observed in sporadic MTC. The identification of RET mutations is relevant to the early diagnosis of hereditary MTC and the prognosis of sporadic MTC. The diagnostic and prognostic role of the RET/PTC rearrangements in PTC is less relevant but still important in patient management, particularly for deciding if a targeted therapy should be initiated. In this Review, we discuss the pathogenic, diagnostic and prognostic roles of the RET proto-oncogene in both PTC and MTC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pellegriti, G., Frasca, F., Regalbuto, C., Squatrito, S. & Vigneri, R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 965212 (2013).
Brito, J. P. & Davies, L. Is there really an increased incidence of thyroid cancer? Curr. Opin. Endocrinol. Diabetes Obes. 21, 405–408 (2014).
Enewold, L. et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol. Biomarkers Prev. 18, 784–791 (2009).
Morris, L. G. & Myssiorek, D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am. J. Surg. 200, 454–461 (2010).
Elisei, R. & Pinchera, A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat. Rev. Endocrinol. 8, 466–475 (2012).
DeLellis, R. A. & Williams, E. D. in World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs (eds DeLellis, R. A. et al.) 51–56 (IARC Press, 2004).
Lodish, M. B. & Stratakis, C. A. RET oncogene in MEN2, MEN2B, MTC and other forms of thyroid cancer. Expert Rev. Anticancer Ther. 8, 625–632 (2008).
Romei, C., Pardi, E., Cetani, F. & Elisei, R. Genetic and clinical features of multiple endocrine neoplasia types 1 and 2. J. Oncol. 2012, 705036 (2012).
Wohllk, N. et al. Multiple endocrine neoplasia type 2. Best Pract. Res. Clin. Endocrinol. Metab. 24, 371–387 (2010).
Grieco, M. et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 60, 557–563 (1990).
Mulligan, L. M. et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363, 458–460 (1993).
Eng, C. et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum. Mol. Genet. 3, 237–241 (1994).
Takahashi, M., Ritz, J. & Cooper, G. M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 42, 581–588 (1985).
Arighi, E., Borrello, M. G. & Sariola, H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 16, 441–467 (2005).
Anders, J., Kjar, S. & Ibanez, C. F. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J. Biol. Chem. 276, 35808–35817 (2001).
Ibanez, C. F. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb. Perspect. Biol. http://dx.doi.org/10.1101/cshperspect.a009134 (2013).
Goodman, K. M. et al. RET recognition of GDNF-GFRα1 ligand by a composite binding site promotes membrane-proximal self-association. Cell Rep. 8, 1894–1904 (2014).
Pasini, B. et al. The physical map of the human RET proto-oncogene. Oncogene 11, 1737–1743 (1995).
Myers, S. M., Eng, C., Ponder, B. A. & Mulligan, L. M. Characterization of RET proto-oncogene 3′ splicing variants and polyadenylation sites: a novel C-terminus for RET. Oncogene 11, 2039–2045 (1995).
Richardson, D. S. et al. Alternative splicing results in RET isoforms with distinct trafficking properties. Mol. Biol. Cell 23, 3838–3850 (2012).
Takahashi, M. et al. Characterization of the ret proto-oncogene products expressed in mouse L cells. Oncogene 8, 2925–2929 (1993).
Baloh, R. H., Enomoto, H., Johnson, E. M. Jr & Milbrandt, J. The GDNF family ligands and receptors — implications for neural development. Curr. Opin. Neurobiol. 10, 103–110 (2000).
Baloh, R. H. et al. GFRα3 is an orphan member of the GDNF/neurturin/persephin receptor family. Proc. Natl Acad. Sci. USA 95, 5801–5806 (1998).
Jing, S. et al. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell 85, 1113–1124 (1996).
Schuchardt, A., D'Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994).
Santoro, M., Melillo, R. M., Carlomagno, F., Vecchio, G. & Fusco, A. Minireview: RET: normal and abnormal functions. Endocrinology 145, 5448–5451 (2004).
Takaya, K. et al. Expression of the RET proto-oncogene in normal human tissues, pheochromocytomas, and other tumors of neural crest origin. J. Mol. Med. (Berl.) 74, 617–621 (1996).
Kimura, E. T. et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC−RAS−BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 63, 1454–1457 (2003).
Ciampi, R. et al. Oncogenic AKAP9−BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Invest. 115, 94–101 (2005).
Fusco, A. et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature 328, 170–172 (1987).
Uchino, S. et al. Somatic mutations in RET exons 12 and 15 in sporadic medullary thyroid carcinomas: different spectrum of mutations in sporadic type from hereditary type. Jpn J. Cancer Res. 90, 1231–1237 (1999).
Eng, C. et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 276, 1575–1579 (1996).
Cerrato, A., De Falco, V. & Santoro, M. Molecular genetics of medullary thyroid carcinoma: the quest for novel therapeutic targets. J. Mol. Endocrinol. 43, 143–155 (2009).
Romei, C. et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC). Clin. Endocrinol. (Oxf.) 74, 241–247 (2011).
Wells, S. A. Jr, Pacini, F., Robinson, B. G. & Santoro, M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J. Clin. Endocrinol. Metab. 98, 3149–3164 (2013).
Elisei, R. et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J. Clin. Endocrinol. Metab. 92, 4725–4729 (2007).
Niccoli-Sire, P. et al. Familial medullary thyroid carcinoma with noncysteine ret mutations: phenotype−genotype relationship in a large series of patients. J. Clin. Endocrinol. Metab. 86, 3746–3753 (2001).
Margraf, R. L. et al. Multiple endocrine neoplasia type 2 RET protooncogene database: repository of MEN2-associated RET sequence variation and reference for genotype/phenotype correlations. Hum. Mutat. 30, 548–556 (2009).
Gimm, O. et al. Germline dinucleotide mutation in codon 883 of the RET proto-oncogene in multiple endocrine neoplasia type 2B without codon 918 mutation. J. Clin. Endocrinol. Metab. 82, 3902–3904 (1997).
Cosci, B. et al. In silico and in vitro analysis of rare germline allelic variants of RET oncogene associated with medullary thyroid cancer. Endocr. Relat. Cancer 18, 603–612 (2011).
Prazeres, H. et al. In vitro transforming potential, intracellular signaling properties, and sensitivity to a kinase inhibitor (sorafenib) of RET proto-oncogene variants Glu511Lys, Ser649Leu, and Arg886Trp. Endocr. Relat. Cancer 18, 401–412 (2011).
Machens, A., Hauptmann, S. & Dralle, H. Modification of multiple endocrine neoplasia 2A phenotype by cell membrane proximity of RET mutations in exon 10. Endocr. Relat. Cancer 16, 171–177 (2009).
Frank-Raue, K. et al. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum. Mutat. 32, 51–58 (2011).
Bihan, H. et al. The clinical spectrum of RET proto-oncogene mutations in codon 790. Eur. J. Endocrinol. 169, 271–276 (2013).
Frank-Raue, K. et al. Difference in development of medullary thyroid carcinoma among carriers of RET mutations in codons 790 and 791. Clin. Endocrinol. (Oxf.) 69, 259–263 (2008).
Erlic, Z. et al. Pathogenicity of DNA variants and double mutations in multiple endocrine neoplasia type 2 and von Hippel−Lindau syndrome. J. Clin. Endocrinol. Metab. 95, 308–313 (2010).
Toledo, R. A. et al. Comprehensive assessment of the disputed RET Y791F variant shows no association with medullary thyroid carcinoma susceptibility. Endocr. Relat. Cancer 22, 65–76 (2015).
Elisei, R. et al. Identification of a novel point mutation in the RET gene (Ala883Thr), which is associated with medullary thyroid carcinoma phenotype only in homozygous condition. J. Clin. Endocrinol. Metab. 89, 5823–5827 (2004).
Romei, C. et al. Twenty years of lesson learning: how does the RET genetic screening test impact the clinical management of medullary thyroid cancer? Clin. Endocrinol. (Oxf.) 82, 892–899 (2015).
Orgiana, G. et al. A new germline RET mutation apparently devoid of transforming activity serendipitously discovered in a patient with atrophic autoimmune thyroiditis and primary ovarian failure. J. Clin. Endocrinol. Metab. 89, 4810–4816 (2004).
Karki, R., Pandya, D., Elston, R. C. & Ferlini, C. Defining 'mutation' and 'polymorphism' in the era of personal genomics. BMC Med. Genom. 8, 37 (2015).
Crockett, D. K. et al. Predicting phenotypic severity of uncertain gene variants in the RET proto-oncogene. PLoS ONE 6, e18380 (2011).
Miyauchi, A. et al. Two germline missense mutations at codons 804 and 806 of the RET proto-oncogene in the same allele in a patient with multiple endocrine neoplasia type 2B without codon 918 mutation. Jpn J. Cancer Res. 90, 1–5 (1999).
Menko, F. H. et al. Atypical MEN type 2B associated with two germline RET mutations on the same allele not involving codon 918. J. Clin. Endocrinol. Metab. 87, 393–397 (2002).
Tessitore, A. et al. A novel case of multiple endocrine neoplasia type 2A associated with two de novo mutations of the RET protooncogene. J. Clin. Endocrinol. Metab. 84, 3522–3527 (1999).
Machens, A. et al. Early malignant progression of hereditary medullary thyroid cancer. N. Engl. J. Med. 349, 1517–1525 (2003).
Frank-Raue, K. et al. Mutations of the ret protooncogene in German multiple endocrine neoplasia families: relation between genotype and phenotype. German Medullary Thyroid Carcinoma Study Group. J. Clin. Endocrinol. Metab. 81, 1780–1783 (1996).
Romei, C. et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur. J. Endocrinol. 163, 301–308 (2010).
Ji, J. H. et al. Identification of driving ALK fusion genes and genomic landscape of medullary thyroid cancer. PLoS Genet. 11, e1005467 (2015).
Romei, C. et al. Low prevalence of the somatic M918T RET mutation in micro-medullary thyroid cancer. Thyroid 22, 476–481 (2012).
Ciampi, R. et al. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid 23, 50–57 (2013).
Moura, M. M., Cavaco, B. M., Pinto, A. E. & Leite, V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J. Clin. Endocrinol. Metab. 96, E863–E868 (2011).
Oriola, J., Halperin, I., Rivera-Fillat, F. & Donis-Keller, H. The finding of a somatic deletion in RET exon 15 clarified the sporadic nature of a medullary thyroid carcinoma suspected to be familial. J. Endocrinol. Invest. 25, 25–31 (2002).
Moura, M. M., Cavaco, B. M. & Leite, V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr. Relat. Cancer 22, R235–R252 (2015).
Simbolo, M. et al. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch. 465, 73–78 (2014).
Goutas, N. et al. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res. 28, 305–308 (2008).
Ceccherini, I. et al. Somatic in frame deletions not involving juxtamembranous cysteine residues strongly activate the RET proto-oncogene. Oncogene 14, 2609–2612 (1997).
Marsh, D. J. et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin. Endocrinol. (Oxf.) 44, 249–257 (1996).
Kato, M. et al. Molecular mechanism of activation and superactivation of Ret tyrosine kinases by ultraviolet light irradiation. Antioxid. Redox Signal. 2, 841–849 (2000).
Kato, M. et al. Ultraviolet light induces redox reaction-mediated dimerization and superactivation of oncogenic Ret tyrosine kinases. Mol. Biol. Cell 11, 93–101 (2000).
Santoro, M. et al. Molecular characterization of RET/PTC3; a novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene 9, 509–516 (1994).
Greco, A., Borrello, M. G., Miranda, C., Degl'Innocenti, D. & Pierotti, M. A. Molecular pathology of differentiated thyroid cancer. Q. J. Nucl. Med. Mol. Imaging 53, 440–453 (2009).
Santoro, M. et al. Development of thyroid papillary carcinomas secondary to tissue-specific expression of the RET/PTC1 oncogene in transgenic mice. Oncogene 12, 1821–1826 (1996).
Santoro, M., Melillo, R. M. & Fusco, A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur. J. Endocrinol. 155, 645–653 (2006).
Bongarzone, I. et al. Molecular characterization of a thyroid tumor-specific transforming sequence formed by the fusion of ret tyrosine kinase and the regulatory subunit RI alpha of cyclic AMP-dependent protein kinase A. Mol. Cell. Biol. 13, 358–366 (1993).
Fugazzola, L. et al. Molecular and biochemical analysis of RET/PTC4, a novel oncogenic rearrangement between RET and ELE1 genes, in a post-Chernobyl papillary thyroid cancer. Oncogene 13, 1093–1097 (1996).
Klugbauer, S., Demidchik, E. P., Lengfelder, E. & Rabes, H. M. Detection of a novel type of RET rearrangement (PTC5) in thyroid carcinomas after Chernobyl and analysis of the involved RET-fused gene RFG5. Cancer Res. 58, 198–203 (1998).
Klugbauer, S. & Rabes, H. M. The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene 18, 4388–4393 (1999).
Nakata, T. et al. Fusion of a novel gene, ELKS, to RET due to translocation t(10;12)(q11;p13) in a papillary thyroid carcinoma. Genes Chromosomes Cancer 25, 97–103 (1999).
Salassidis, K. et al. Translocation t(10;14)(q11.2:q22.1) fusing the kinetin to the RET gene creates a novel rearranged form (PTC8) of the RET proto-oncogene in radiation-induced childhood papillary thyroid carcinoma. Cancer Res. 60, 2786–2789 (2000).
Klugbauer, S., Jauch, A., Lengfelder, E., Demidchik, E. & Rabes, H. M. A novel type of RET rearrangement (PTC8) in childhood papillary thyroid carcinomas and characterization of the involved gene (RFG8). Cancer Res. 60, 7028–7032 (2000).
Corvi, R., Berger, N., Balczon, R. & Romeo, G. RET/PCM-1: a novel fusion gene in papillary thyroid carcinoma. Oncogene 19, 4236–4242 (2000).
Saenko, V. et al. Novel tumorigenic rearrangement, Δrfp/ret, in a papillary thyroid carcinoma from externally irradiated patient. Mutat. Res. 527, 81–90 (2003).
Ciampi, R., Giordano, T. J., Wikenheiser-Brokamp, K., Koenig, R. J. & Nikiforov, Y. E. HOOK3-RET: a novel type of RET/PTC rearrangement in papillary thyroid carcinoma. Endocr. Relat. Cancer 14, 445–452 (2007).
Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159, 676–690 (2014).
Hamatani, K. et al. A novel RET rearrangement (ACBD5/RET) by pericentric inversion, inv(10)(p12.1;q11.2), in papillary thyroid cancer from an atomic bomb survivor exposed to high-dose radiation. Oncol. Rep. 32, 1809–1814 (2014).
Klugbauer, S., Demidchik, E. P., Lengfelder, E. & Rabes, H. M. Molecular analysis of new subtypes of ELE/RET rearrangements, their reciprocal transcripts and breakpoints in papillary thyroid carcinomas of children after Chernobyl. Oncogene 16, 671–675 (1998).
Elisei, R. et al. New breakpoints in both the H4 and RET genes create a variant of PTC-1 in a post-Chernobyl papillary thyroid carcinoma. Clin. Endocrinol. (Oxf.) 53, 131–136 (2000).
Caudill, C. M., Zhu, Z., Ciampi, R., Stringer, J. R. & Nikiforov, Y. E. Dose-dependent generation of RET/PTC in human thyroid cells after in vitro exposure to γ-radiation: a model of carcinogenic chromosomal rearrangement induced by ionizing radiation. J. Clin. Endocrinol. Metab. 90, 2364–2369 (2005).
Ameziane-El-Hassani, R. et al. Role of H2O2 in RET/PTC1 chromosomal rearrangement produced by ionizing radiation in human thyroid cells. Cancer Res. 70, 4123–4132 (2010).
Nikiforova, M. N. et al. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science 290, 138–141 (2000).
Gandhi, M., Evdokimova, V. & Nikiforov, Y. E. Mechanisms of chromosomal rearrangements in solid tumors: the model of papillary thyroid carcinoma. Mol. Cell. Endocrinol. 321, 36–43 (2010).
Gandhi, M., Medvedovic, M., Stringer, J. R. & Nikiforov, Y. E. Interphase chromosome folding determines spatial proximity of genes participating in carcinogenic RET/PTC rearrangements. Oncogene 25, 2360–2366 (2006).
Gandhi, M., Evdokimova, V. & Nikiforov, Y. E. Frequency of close positioning of chromosomal loci detected by FRET correlates with their participation in carcinogenic rearrangements in human cells. Genes Chromosomes Cancer 51, 1037–1044 (2012).
Schneider, A. B. Radiation-induced thyroid tumors. Endocrinol. Metab. Clin. North Am. 19, 495–508 (1990).
Ron, E. et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat. Res. 141, 259–277 (1995).
Cardis, E. et al. Cancer consequences of the Chernobyl accident: 20 years on. J. Radiol. Prot. 26, 127–140 (2006).
Cardis, E. et al. Risk of thyroid cancer after exposure to 131I in childhood. J. Natl Cancer Inst. 97, 724–732 (2005).
Gandhi, M., Dillon, L. W., Pramanik, S., Nikiforov, Y. E. & Wang, Y. H. DNA breaks at fragile sites generate oncogenic RET/PTC rearrangements in human thyroid cells. Oncogene 29, 2272–2280 (2010).
Elisei, R. et al. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J. Clin. Endocrinol. Metab. 86, 3211–3216 (2001).
Nikiforov, Y. E. RET/PTC rearrangement in thyroid tumors. Endocr. Pathol. 13, 3–16 (2002).
Ricarte-Filho, J. C. et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J. Clin. Invest. 123, 4935–4944 (2013).
Nikiforov, Y. E., Rowland, J. M., Bove, K. E., Monforte-Munoz, H. & Fagin, J. A. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 57, 1690–1694 (1997).
Fenton, C. L. et al. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J. Clin. Endocrinol. Metab. 85, 1170–1175 (2000).
Jarzab, B. & Handkiewicz-Junak, D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens) 6, 200–209 (2007).
Rabes, H. M. et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res. 6, 1093–1103 (2000).
Romei, C. et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J. Clin. Endocrinol. Metab. 97, E1758–E1765 (2012).
Smyth, P. et al. ret/PTC and BRAF act as distinct molecular, time-dependant triggers in a sporadic Irish cohort of papillary thyroid carcinoma. Int. J. Surg. Pathol. 13, 1–8 (2005).
Domingues, R., Mendonca, E., Sobrinho, L. & Bugalho, M. J. Searching for RET/PTC rearrangements and BRAF V599E mutation in thyroid aspirates might contribute to establish a preoperative diagnosis of papillary thyroid carcinoma. Cytopathology 16, 27–31 (2005).
Guerra, A. et al. Prevalence of RET/PTC rearrangement in benign and malignant thyroid nodules and its clinical application. Endocr. J. 58, 31–38 (2011).
Rhoden, K. J. et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto's thyroiditis share low-level recombination events with a subset of papillary carcinoma. J. Clin. Endocrinol. Metab. 91, 2414–2423 (2006).
Sheils, O. M. et al. ret/PTC-1 activation in Hashimoto thyroiditis. Int. J. Surg. Pathol. 8, 185–189 (2000).
Wirtschafter, A. et al. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis. Laryngoscope 107, 95–100 (1997).
Ishizaka, Y. et al. Detection of retTPC/PTC transcripts in thyroid adenomas and adenomatous goiter by an RT-PCR method. Oncogene 6, 1667–1672 (1991).
Bounacer, A. et al. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene 15, 1263–1273 (1997).
Zhu, Z., Ciampi, R., Nikiforova, M. N., Gandhi, M. & Nikiforov, Y. E. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J. Clin. Endocrinol. Metab. 91, 3603–3610 (2006).
Nikiforov, Y. E. RET/PTC rearrangement — a link between Hashimoto's thyroiditis and thyroid cancer...or not. J. Clin. Endocrinol. Metab. 91, 2040–2042 (2006).
Liu, Z. et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J. Clin. Endocrinol. Metab. 93, 3106–3116 (2008).
Mochizuki, K. et al. RET rearrangements and BRAF mutation in undifferentiated thyroid carcinomas having papillary carcinoma components. Histopathology 57, 444–450 (2010).
Sheils, O. M., O'Leary, J. J. & Sweeney, E. C. Assessment of ret/PTC-1 rearrangements in neoplastic thyroid tissue using TaqMan RT-PCR. J. Pathol. 192, 32–36 (2000).
Ricarte-Filho, J. C. et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 69, 4885–4893 (2009).
Tallini, G. et al. RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin. Cancer Res. 4, 287–294 (1998).
Soares, P., Fonseca, E., Wynford-Thomas, D. & Sobrinho-Simoes, M. Sporadic ret-rearranged papillary carcinoma of the thyroid: a subset of slow growing, less aggressive thyroid neoplasms? J. Pathol. 185, 71–78 (1998).
Mayr, B. et al. ret/PTC-1, -2, and -3 oncogene rearrangements in human thyroid carcinomas: implications for metastatic potential? J. Clin. Endocrinol. Metab. 82, 1306–1307 (1997).
Grubbs, E. G. et al. RET fusion as a novel driver of medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 100, 788–793 (2015).
Flavin, R. et al. RET/PTC rearrangement occurring in primary peritoneal carcinoma. Int. J. Surg. Pathol. 17, 187–197 (2009).
Ballerini, P. et al. RET fusion genes are associated with chronic myelomonocytic leukemia and enhance monocytic differentiation. Leukemia 26, 2384–2389 (2012).
Bossi, D. et al. Functional characterization of a novel FGFR1OP-RET rearrangement in hematopoietic malignancies. Mol. Oncol. 8, 221–231 (2014).
Kohno, T. et al. KIF5B−RET fusions in lung adenocarcinoma. Nat. Med. 18, 375–377 (2012).
Wang, R. et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 30, 4352–4359 (2012).
Drilon, A. et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 3, 630–635 (2013).
Lira, M. E. et al. A single-tube multiplexed assay for detecting ALK, ROS1, and RET fusions in lung cancer. J. Mol. Diagn. 16, 229–243 (2014).
Nakaoku, T. et al. Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin. Cancer Res. 20, 3087–3093 (2014).
Kebebew, E., Ituarte, P. H., Siperstein, A. E., Duh, Q. Y. & Clark, O. H. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 88, 1139–1148 (2000).
Pelizzo, M. R. et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur. J. Surg. Oncol. 33, 493–497 (2007).
Elisei, R. et al. The timing of total thyroidectomy in RET gene mutation carriers could be personalized and safely planned on the basis of serum calcitonin: 18 years experience at one single center. J. Clin. Endocrinol. Metab. 97, 426–435 (2012).
Pacini, F. et al. Early treatment of hereditary medullary thyroid carcinoma after attribution of multiple endocrine neoplasia type 2 gene carrier status by screening for ret gene mutations. Surgery 118, 1031–1035 (1995).
Lips, C. J. et al. Clinical screening as compared with DNA analysis in families with multiple endocrine neoplasia type 2A. N. Engl. J. Med. 331, 828–835 (1994).
Frilling, A. et al. Presymptomatic genetic screening in families with multiple endocrine neoplasia type 2. J. Mol. Med. (Berl.) 73, 229–233 (1995).
Skinner, M. A. et al. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N. Engl. J. Med. 353, 1105–1113 (2005).
Kloos, R. T. et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19, 565–612 (2009).
Elisei, R., Alevizaki, M., Conte-Devolx, B., Frank-Raue, K. & Lette, V. 2012 European Thyroid Association guidelines for genetic testing and its clinical consequences in medullary thyroid cancer. Eur. Thyroid J. 1, 216–231 (2012).
Baloch, Z. W. & LiVolsi, V. A. Fine-needle aspiration of the thyroid: today and tomorrow. Best Pract. Res. Clin. Endocrinol. Metab. 22, 929–939 (2008).
Cooper, D. S. et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009).
Pacini, F. et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 154, 787–803 (2006).
Cantara, S. et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J. Clin. Endocrinol. Metab. 95, 1365–1369 (2010).
Nikiforov, Y. E. et al. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid 25, 1217–1223 (2015).
Rossi, M. et al. Relevance of BRAFV600E mutation testing versus RAS point mutations and RET/PTC rearrangements evaluation in the diagnosis of thyroid cancer. Thyroid 25, 221–228 (2015).
Elisei, R. et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J. Clin. Endocrinol. Metab. 93, 682–687 (2008).
Moura, M. M. et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br. J. Cancer 100, 1777–1783 (2009).
Mian, C. et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratification. Eur. J. Endocrinol. 164, 971–976 (2011).
Salvatore, D. et al. Increased in vivo phosphorylation of ret tyrosine 1062 is a potential pathogenetic mechanism of multiple endocrine neoplasia type 2B. Cancer Res. 61, 1426–1431 (2001).
Russo, R. et al. MEN IIB. A case report [Italian]. Radiol. Med. 87, 168–171 (1994).
Wells, S. A. Jr. et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25, 567–610 (2015).
Thomas, G. A. et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J. Clin. Endocrinol. Metab. 84, 4232–4238 (1999).
Romei, C. et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr. Relat. Cancer 15, 511–520 (2008).
Jhiang, S. M. & Mazzaferri, E. L. The RET/PTC oncogene in papillary thyroid carcinoma. J. Lab. Clin. Med. 123, 331–337 (1994).
Sugg, S. L. et al. Oncogene profile of papillary thyroid carcinoma. Surgery 125, 46–52 (1999).
Yip, L. et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: a study of 1510 patients. Ann. Surg. 262, 519–525 (2015).
Gharib, H. et al. Medullary thyroid carcinoma: clinicopathologic features and long-term follow-up of 65 patients treated during 1946 through 1970. Mayo Clin. Proc. 67, 934–940 (1992).
Klein Hesselink, E. N. et al. Therapy of endocrine disease: response and toxicity of small-molecule tyrosine kinase inhibitors in patients with thyroid carcinoma: a systematic review and meta-analysis. Eur. J. Endocrinol. 172, R215–R225 (2015).
Carlomagno, F. et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 62, 7284–7290 (2002).
Sherman, S. I. Targeted therapies for thyroid tumors. Mod. Pathol. 24, S44–S52 (2011).
Schlumberger, M. & Sherman, S. I. Approach to the patient with advanced differentiated thyroid cancer. Eur. J. Endocrinol. 166, 5–11 (2012).
Elisei, R. et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 31, 3639–3646 (2013).
Wells, S. A. Jr. et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012).
Brose, M. S. et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384, 319–328 (2014).
Schlumberger, M. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 (2015).
Leboulleux, S. et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 13, 897–905 (2012).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Romei, C., Ciampi, R. & Elisei, R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 12, 192–202 (2016). https://doi.org/10.1038/nrendo.2016.11
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.11
This article is cited by
-
RET/PTC rearrangement in papillary thyroid carcinoma arising in malignant struma ovarii with abdominal wall metastasis and cervical thyroid gland: a case report and review of the literature
Thyroid Research (2023)
-
Will metformin use lead to a decreased risk of thyroid cancer? A systematic review and meta-analyses
European Journal of Medical Research (2023)
-
Genetically engineered mouse models of head and neck cancers
Oncogene (2023)
-
Insights into pralsetinib resistance to the non-gatekeeper RET kinase G810C mutation through molecular dynamics simulations
Journal of Molecular Modeling (2023)
-
Update on C-Cell Neuroendocrine Neoplasm: Prognostic and Predictive Histopathologic and Molecular Features of Medullary Thyroid Carcinoma
Endocrine Pathology (2023)