Key Points
-
The disease process leading to type 1 diabetes mellitus (T1DM) is, in many cases, initiated during the first few years of life, when the intestinal microbiota undergoes dynamic development
-
The available data are insufficient to assess whether alterations in the gut microbiota are involved in the initiation of T1DM
-
After the appearance of the first disease-predictive autoantibodies, children who progress to clinical T1DM have a reduced bacterial diversity and a decreased abundance of bacteria that produce butyrate or lactate
-
The mechanisms by which intestinal microorganisms might affect the initiation of β-cell autoimmunity and the progression from seroconversion to clinical disease need to be identified
-
Standard operating procedures should be applied for the sampling, handling and storage of stool samples, as well as for DNA extraction, to minimize the effect of technical biases
Abstract
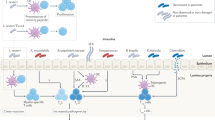
Type 1 diabetes mellitus (T1DM) is a chronic immune-mediated disease with a subclinical prodromal period, characterized by selective loss of insulin-producing-β cells in the pancreatic islets of genetically susceptible individuals. The incidence of T1DM has increased several fold in most developed countries since World War II, in conjunction with other immune-mediated diseases. Rapid environmental changes and modern lifestyles are probably the driving factors that underlie this increase. These effects might be mediated by changes in the human microbiota, particularly the intestinal microbiota. Research on the gut microbiome of individuals at risk of developing T1DM and in patients with established disease is still in its infancy, but initial findings indicate that the intestinal microbiome of individuals with prediabetes or diabetes mellitus is different to that of healthy individuals. The gut microbiota in individuals with preclinical T1DM is characterized by Bacteroidetes dominating at the phylum level, a dearth of butyrate-producing bacteria, reduced bacterial and functional diversity and low community stability. However, these changes seem to emerge after the appearance of autoantibodies that are predictive of T1DM, which suggests that the intestinal microbiota might be involved in the progression from β-cell autoimmunity to clinical disease rather than in the initiation of the disease process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Knip, M. et al. Environmental triggers and determinants of β-cell autoimmunity and type 1 diabetes. Diabetes 54, S125–S136 (2005).
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. Lancet 383, 69–82 (2014).
Knip, M. Pathogenesis of type 1 diabetes: implications for incidence trends. Horm. Res. Paediatr. 76 (Suppl. 1), 57–64 (2011).
Harjutsalo, V., Sund, R., Knip, M. & Groop, P. H. Incidence of type 1 diabetes in Finland. JAMA 310, 427–428 (2013).
Okada, H., Kuhn, C., Feillet, H. & Bach, J. F. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin. Exp. Immunol. 160, 1–9 (2010).
Kondrashova, A., Seiskari, T., Ilonen, J., Knip, M. & Hyöty, H. The 'hygiene hypothesis' and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. APMIS 121, 478–493 (2013).
von Hertzen, L. et al. Helsinki alert of biodiversity and health. Ann. Med. 47, 218–225 (2015).
Quercia, S. et al. From lifetime to evolution: timescales of human gut microbiota adaptation. Front. Microbiol. 5, 587 (2014).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Renz, H., Brandtzaeg, P. & Hornef, M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 12, 9–23 (2012).
Sommer, F. & Bäckhed, F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Garn, H., Neves, J. F., Blumberg, R. S. & Renz, H. Effect of barrier microbes on organ based inflammation. J. Allergy Clin. Immunol. 131, 1465–1478 (2013).
West, C. E., Jenmalm, M. C. & Prescott, S. L. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin. Exp. Allergy 45, 43–53 (2015).
Dunne, J. L. et al. The intestinal microbiome in type 1 diabetes. Clin. Exp. Immunol. 177, 30–37 (2014).
West. C. E. et al. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J. Allergy Clin. Immunol. 135, 3–13 (2015).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Booijink, C. C. et al. High temporal and inter-individual variation detected in the human ileal microbiota. Environ. Microbiol. 12, 3213–3227 (2010).
El-Aidy, S., van der Bogert, B. & Kleerebezem, M. The small intestine microbiota, nutritional modulation and relevance for health. Curr. Opin. Biotechnol. 32, 14–20 (2015).
Franzosa, E. A. et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl Acad. Sci. USA 111, E2329–E2338 (2014).
Goodrich, J. K. et al. Conducting a microbiome study. Cell 158, 250–262 (2014).
Fraher, M. H., O'Toole, P. W. & Quigley, E. M. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat. Rev. Gastroenterol. Hepatol. 9, 312–322 (2012).
Jumpstart Consortium Human Microbiome Project Data Generation Working Group. Evaluation of 16S rDNA-based community profiling for human microbiome research. PLoS ONE 7, e39315 (2012).
Morgan, X. C. & Huttenhower, C. Meta'omic analytic techniques for studying the intestinal microbiome. Gastroenterology 146, 1437–1448 (2014).
Doll, H. M. et al. Utilizing novel diversity estimators to quantify multiple dimensions of microbial biodiversity across domains. BMC Microbiol. 13, 259 (2013).
Rajilic-Stojanovic, M. et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ. Microbiol. 11, 1736–1751 (2009).
Lee, C. K. et al. Groundtruthing next-gen sequencing for microbial ecology-biases and errors in community structure estimates from PCR amplicon pyrosequencing. PLoS ONE 7, e44224 (2012).
Langille, M. G. et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821 (2013).
Franzosa, E. A. et al. Sequencing and beyond: integrating molecular 'omics' for microbial community profiling. Nat. Rev. Microbiol. 13, 360–372 (2015).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072 (2006).
Pruesse, E. et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35, 7188–7196 (2007).
Sul, W. J. et al. Bacterial community comparisons by taxonomy-supervised analysis independent of sequence alignment and clustering. Proc. Natl Acad. Sci. USA 108, 14637–14642 (2011).
Lozupone, C. A. et al. Meta-analyses of studies of the human microbiota. Genome Res. 23, 1704–1714 (2013).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Song, S. J. et al. Cohabiting family members share microbiota with one another and with their dogs. eLIFE 2, e00458 (2013).
Koren, O. et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 (2012).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra65 (2014).
Matamoro, S., Gras-Leguen, C., Le Vacon, F., Potel, G. & de La Cochetiere, M. F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 21, 167–173 (2013).
Mackie, R. I., Sghir, A. & Gaskins, H. R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69, S1035–S1045 (1999).
Khafipour, E. & Ghia, J. E. Mode of delivery and inflammatory disorders. J. Immunol. Clin. Res. 1, 1004 (2013).
Dominguez-Bello, M. G., Blaser, M. J., Ley, R. E. & Knight, R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 140, 1713–1719 (2011).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Biasucci, G., Benenati, B., Morelli, L., Bessi, E. & Boehm, G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J. Nutr. 138, 1796S–1800S (2008).
Jakobsson, H. E. et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonization and reduced Th1 responses in infants delivered by caesarean section. Gut 63, 559–566 (2014).
Penders, J. et al.Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 132, 601–607 (2013).
Neu, J. & Rushing, J. Caesarean versus vaginal delivery: long term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 38, 321–331 (2011).
Grönlund, M. M., Grzeskowiak, L., Isolauri, E. & Salminen, S. Influence of mother's intestinal microbiota on gut colonization in the infant. Gut Microbes 2, 227–233 (2011).
Ege, M. J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364, 701–709 (2011).
Sjögren, Y. M., Jenmalm, M. C., Böttcher, M. F., Björkstén, B. & Sverremark-Ekström, E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy 39, 518–526 (2009).
Hanski, I. et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl Acad. Sci. USA 109, 8334–8339 (2012).
Hollister, E. B., Gao, C. & Versalovic, J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146, 1449–1458 (2014).
Borre, Y. E., Moloney, R. D., Clarke, G., Dinan, T. G. & Cryan, J. F. The impact of microbiota on brain and behavior: mechanisms and therapeutic potential. Adv. Exp. Med. Biol. 817, 373–403 (2014).
Jeffery, I. B. et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 (2012).
Stark, P. L. & Lee, A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year. J. Med. Microbiol. 15, 189–203 (1982).
Jost, T., Lacroix, C., Braegger, C. P., Rochat, F. & Chassard, C. Vertical mother−neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 3, 203–220 (2013).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Kramer, M. S. Breastfeeding and allergy: the evidence. Ann. Nutr. Metab. 59 (Suppl. 1), 20–26 (2011).
Elenberg, Y. & Shaoul, R. The role of infant nutrition in the prevention of future disease. Front. Pediatr. 2, 73 (2014).
Robinson, S. & Fall, C. Infant nutrition and later health: a review of current evidence. Nutrients 4, 859–874 (2012).
Ballard, O. & Morrow, A. L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 60, 49–74 (2013).
Donnet-Hughes, A. et al. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc. Nutr. Soc. 69, 407–415 (2010).
Zivkovic, A. M., German, J. B., Lebrilla, C. B. & Mills, D. A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl Acad. Sci. USA 108, 4653–4658 (2011).
Garrido, D., Barile, D. & Mills, D. A. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv. Nutr. 3, 415S–421S (2012).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Lee, S., Sung, J., Lee, J. & Ko, G. Comparison of the gut microbiotas of healthy adult twins living in South Korea and the United States. Appl. Environ. Microbiol. 77, 7433–7437 (2011).
Maurice, C. F., Haiser, H. J. & Turnbaugh, P. J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 152, 39–50 (2013).
Vanhoutte, T., Huys, G., Brandt, E. & Swings, J. Temporal stability analysis of the microbiota in human feces by denaturing gradient gel electrophoresis using universal and group-specific 16S rRNA gene primers. FEMS Microbiol. Ecol. 48, 437–446 (2004).
Tannock, G. W. et al. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 66, 2578–2588 (2000).
Zhao, J., Murray, S. & Lipuma, J. J. Modeling the impact of antibiotic exposure on human microbiota. Sci. Rep. 4, 4345 (2014).
Kemppainen, K. M. et al. Early childhood gut microbiomes show strong geographical differences among subjects at high risk for type 1 diabetes. Diabetes Care 38, 329–332 (2015).
The TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr. Diabetes 8, 249–348 (2007).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Ottman, N., Smidt, H., de Vos, W. M. & Belzer, C. The function of our microbiota: who is out there and what do they do? Front. Cell. Infect. Microbiol. 2, 104 (2012).
Vaarala, O. Is the origin of type 1 diabetes in the gut. Immunol. Cell Biol. 90, 271–278 (2012).
Whitacre, C. C. Sex differences in autoimmune disease. Nat. Immunol. 2, 777–780 (2001).
Gale, E. A. M. & Gillespie, K. M. Diabetes and gender. Diabetologia 44, 3–15 (2001).
Mueller, S. et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72, 1027–1033 (2006).
Markle, J. G. M. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013).
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412 (2013).
Serrezze, D. V. & Chen, Y. G. Of mice and men: use of animal models to identify possible interventions for the prevention of autoimmune diabetes in humans. Trends Immunol. 26, 603–607 (2005).
Gonzalez, A. et al. Genetic control of diabetes progression. Immunity 7, 873–883 (1997).
Wong, F. S. et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 5, 1026–1031 (1999).
Tisch, R. et al. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 366, 72–75 (1993).
Garchon, H. J., Bedossa, P., Eloy, L. & Bach, J. F. Identification and mapping to chromosome 1 of a susceptibility locus for periinsulitis in non-obese diabetic mice. Nature 353, 260–262 (1991).
Reddy, S., Bibby, N. J. & Elliott, R. B. Ontogeny of islet cell antibodies, insulin autoantibodies and insulitis in the non-obese diabetic mouse. Diabetologia 31, 322–328 (1988).
Shimada, A., Charlton, B., Taylor-Edwards, C. & Fathman, C. G. β-cell destruction may be a late consequence of the autoimmune process in nonobese diabetic mice. Diabetes 45, 1063–1067 (1996).
Greiner, T. U. et al. The gut microbiota modulates glycemic control and the serum metabolite profile in non-obese diabetic mice. PLoS ONE 9, e110359 (2014).
Wen, L. et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455, 1109–1113 (2008).
Peng, J. et al. Long-term effect of gut microbiota transfer on diabetes development. J. Autoimmun. 53, 85–94 (2014).
King, C. & Sarvetnick, N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS ONE 6, e17049 (2011).
Kriegel, M. A. et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 108, 11548–11553 (2011).
Hansen, C. H. et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 55, 2285–2294 (2012).
Alam, C. et al. Inflammatory tendencies and overproduction of IL-17 in the colon of young NOD mice are counteracted with diet change. Diabetes 59, 2237–2246 (2010).
Emani, R. et al. Peritoneal cavity is a route for gut-derived microbial signals to promote autoimmunity in non-obese diabetic mice. Scand. J. Immunol. 81, 102–109 (2015).
Hansen, C. H. et al. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes 63, 2821–2832 (2014).
Marietta, E. V. et al. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS ONE 8, e78687 (2013).
Toivonen, R. K. et al. Fermentable fibres condition colon microbiota and promote diabetogenesis in NOD mice. Diabetologia 57, 2183–2192 (2014).
Wolf, K. J. et al. Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J. Histochem. Cytochem. 62, 237–250 (2014).
Roep, B. O. & Atkinson, M. Animal models have little to teach us about type 1 diabetes: 1. In support of this proposal. Diabetologia 47, 1650–1656 (2004).
Kimpimäki, T. et al. The first signs of ß-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J. Clin. Endocrinol. Metab. 86, 4782–4788 (2001).
Parikka, V. et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 55, 1926–1936 (2012).
Krischer, J. P. et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 58, 980–987 (2015).
Kostic, A. D. et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 17, 260–273 (2015).
Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5, 82–91 (2011).
Kupila, A. et al. Feasibility of genetic and immunological prediction of type 1 diabetes in a population-based birth cohort. Diabetologia 44, 290–297 (2001).
Brown, C. T. et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 6, e25792 (2011).
Knip, M. et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N. Engl. J. Med. 363, 1900–1908 (2010).
Vaarala, O. et al. Removal of bovine insulin from cow's milk formula and early initiation of beta-cell autoimmunity. Arch. Pediatr. Adolesc. Med. 166, 608–614 (2012).
de Goffau, M. C. et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 62, 1238–1244 (2013).
Hummel, S., Pflüger, M., Hummel, M., Bonifacio, E. & Ziegler, A. G. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 34, 1301–1305 (2011).
Endesfelder, D. et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes 63, 2006–2014 (2014).
Davis-Richardson, A. et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 5, 678 (2014).
Leonard, M. T. et al. The methylome of the gut microbiome: disparate Dam methylation patterns in intestinal Bacteroides dorei. Front. Microbiol. 5, 361 (2014).
Kallionpää, H. et al. The standard of hygiene and immune adaptation in newborn infants. Clin. Immunol. 155, 136–147 (2014).
Alkanani, A. K. et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64, 3510–3520 (2015).
Murri, M. et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 11, 46 (2013).
Mejía-León, M. E., Petrosino, J. F., Ajami, N. J., Domínguez-Bello, M. G. & de la Barca, A. M. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 4, 3814 (2014).
Hague, A., Butt, A. J. & Paraskeva, C. The role of butyrate in human colonic epithelial cells: an energy source or inducer of differentiation and apoptosis? Proc. Nutr. Soc. 55, 937–943 (1996).
Peng, L., Li, Z. R., Green, R. S., Holzman, I. R. & Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139, 1619–1625 (2009).
Vaarala, O., Atkinson, M. A. & Neu, J. The 'perfect storm' for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 57, 2555–2562 (2008).
Hansen, C. H. et al. Mode of delivery shapes gut colonization pattern and modulates regulatory immunity in mice. J. Immunol. 193, 1213–1222 (2014).
de Goffau, M. C. et al. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 57, 1569–1577 (2014).
Patil, K. R. et al. Taxonomic metagenome sequence assignment with structured output models. Nat. Methods 8, 191–192 (2011).
Huson, D. H., Auch, A. F., Qi, J. & Schuster, S. C. MEGAN analysis of metagenomic data. Genome Res. 17, 377–386 (2007).
Segata, N. et al. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 9, 811–814 (2012).
Martin, J. et al. Optimizing read mapping to reference genomes to determine composition and species prevalence in microbial communities. PLoS ONE 7, e36427 (2012).
Luo, C. et al. Strain profiling and genotyping of microbial communities from metagenomic sequence data. Nat. Biotechnol. 33, 1045–1052 (2015).
Li, R. et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 20, 265–272 (2010).
Zerbino, D. R. & Birney, E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18, 821–829 (2008).
Namiki, T., Hachiya, T., Tanaka, H. & Sakakibara, Y. MetaVelvet: an extension of Velvet assembler to de novo metagenome assembly from short sequence reads. Nucleic Acids Res. 40, e155 (2012).
Kultima, J. R. et al. MOCAT: a metagenomics assembly angene prediction toolkit. PLoS ONE 7, e47656 (2012).
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Kanehisa, M., Goto, S., Furumichi, M., Tanabe, M. & Hirakawa, M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 38, D355–D360 (2010).
Tatusov, R. L. et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, 41 (2003).
Punta, M. et al. The Pfam protein families database. Nucleic Acids Res. 40, D290–D301 (2012).
Cantarel, B. L. et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 37, D233–238 (2009).
Arumugam, M., Harrington, E. D., Foerstner, K. U., Raes, J. & Bork, P. Smash-Community: a metagenomic annotation and analysis tool. Bioinformatics 26, 2977–2978 (2010).
Abubucker, S. et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput. Biol. 8, e1002358 (2012).
Sanli, K., Karlsson, F. H., Nookaew, I. & Nielsen, J. FANTOM: functional and taxonomic analysis of metagenomes. BMC Bioinformatics 14, 38 (2013).
Seshadri, R., Kravitz, S. A., Smarr, L., Gilna, P. & Frazier, M. CAMERA: a community resource for metagenomics. PLoS Biol. 5, e75 (2007).
Markowitz, V. M. et al. IMG/M: a data management and analysis system for metagenomes. Nucleic Acids Res. 36, D534–D538 (2008).
Meyer, F. et al. The metagenomics RAST server — a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 9, 386 (2008).
Acknowledgements
M.K. and H.S. acknowledge support from the Finnish Centre of Excellence in Molecular Systems Immunology and Physiology Research 2012–17 (Academy of Finland, Decision No. 250114).
Author information
Authors and Affiliations
Contributions
M.K. and H.S. researched data for the article, provided substantial contributions to the discussion of content, wrote the manuscript and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Knip, M., Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol 12, 154–167 (2016). https://doi.org/10.1038/nrendo.2015.218
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.218
This article is cited by
-
Antibiotic exposures and the development of pediatric autoimmune diseases: a register-based case–control study
Pediatric Research (2023)
-
Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis
Bone Research (2023)
-
Chiral coating-mediated interactions of bacteria with diverse biointerfaces
Science China Chemistry (2023)
-
The countdown to type 1 diabetes: when, how and why does the clock start?
Diabetologia (2023)
-
Microbiome composition and central serotonergic activity in patients with depression and type 1 diabetes
European Archives of Psychiatry and Clinical Neuroscience (2023)