Key Points
-
The thymus is the primary lymphoid organ responsible for the generation of T cells
-
Thymus physiology and T-cell development can be controlled by hormones, via a variety of endocrine and paracrine pathways
-
Microenvironmental cells in the thymus constitutively produce hormones that are typically secreted by the pituitary gland, such as growth hormone, prolactin, oxytocin and vasopressin
-
Glucocorticoids induce thymocyte depletion through caspase-dependent apoptosis, whereas growth hormone enhances thymocyte proliferation and migration
-
Considering the variety of the interactions between the endocrine, the nervous and the immune systems, dysfunctions in one of these systems can affect the other
-
Acute infection by Trypanosoma cruzi (the causative agent of Chagas disease) induces thymic atrophy through glucocorticoid-mediated thymocyte depletion, which can be counteracted by exogenous prolactin
Abstract
The physiology of the thymus, the primary lymphoid organ in which T cells are generated, is controlled by hormones. Data from animal models indicate that several peptide and nonpeptide hormones act pleiotropically within the thymus to modulate the proliferation, differentiation, migration and death by apoptosis of developing thymocytes. For example, growth hormone and prolactin can enhance thymocyte proliferation and migration, whereas glucocorticoids lead to the apoptosis of these developing cells. The thymus undergoes progressive age-dependent atrophy with a loss of cells being generated and exported, therefore, hormone-based therapies are being developed as an alternative strategy to rejuvenate the organ, as well as to augment thymocyte proliferation and the export of mature T cells to peripheral lymphoid organs. Some hormones (such as growth hormone and progonadoliberin-1) are also being used as therapeutic agents to treat immunodeficiency disorders associated with thymic atrophy, such as HIV infection. In this Review, we discuss the accumulating data that shows the thymus gland is under complex and multifaceted hormonal control that affects the process of T-cell development in health and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Savino, W. Intrathymic T cell migration is a multivectorial process under a complex neuroendocrine control. Neuroimmunomodulation 17, 142–145 (2010).
Besedovsky, H. O. & Sorkin, E. Thymus involvement in female sexual maturation. Nature 249, 356–358 (1974).
Besedovsky, H., del Rey, A., Sorkin, E. & Dinarello, C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233, 652–654 (1986).
Blalock, J. E., Smith, E. M. & Meyer, W. J. 3rd. The pituitary-adrenocortical axis and the immune system. Clin. Endocrinol. Metab. 14, 1021–1038 (1985).
Savino, W. & Dardenne, M. Immune-neuroendocrine interactions. Immunol. Today 16, 318–322 (1995).
Selye, H. Implications of stress concept. N. Y. State J. Med. 75, 2139–2145 (1975).
Ader, R., Felton, D. L., Cohen, H. (Eds) Psychoneuroimmunology 4th edn (Academic Press, 2006).
Savino, W. & Dardenne, M. Neuroendocrine control of thymus physiology. Endocr. Rev. 21, 412–443 (2000).
Reggiani, P., Martines, E., Ferese, C., Goya, R. & Console, G. Morphological restoration of gonadotrope population by thymulin gene therapy in nude mice. Histol. Histopathol. 24, 729–735 (2009).
de Mello-Coelho, V., Villa-Verde, D. M., Dardenne, M. & Savino, W. Pituitary hormones modulate cell-cell interactions between thymocytes and thymic epithelial cells. J. Neuroimmunol. 76, 39–49 (1997).
Savino, W., Smaniotto, S., Mendes-da-Cruz, D. A. & Dardenne, M. Growth hormone modulates migration of thymocytes and peripheral T cells. Ann. N. Y. Acad. Sci. 1261, 49–54 (2012).
Moll, U. M., Lane, B. L., Robert, F., Geenen, V. & Legros, J. J. The neuroendocrine thymus. Abundant occurrence of oxytocin-, vasopressin-, and neurophysin-like peptides in epithelial cells. Histochemistry 89, 385–390 (1988).
Lind, E. F., Prockop, S. E., Porritt, H. E. & Petrie, H. T. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J. Exp. Med. 194, 127–134 (2001).
Attaf, M., Huseby, E. & Sewell, A. K. αβ T cell receptors as predictors of health and disease. Cell. Mol. Immunol. 12, 391–399 (2015).
Miles, J. J., Douek, D. C. & Price, D. A. Bias in the αβ T-cell repertoire: implications for disease pathogenesis and vaccination. Immunol. Cell Biol. 89, 375–387 (2011).
Godfrey, D. I. & Zlotnik, A. Control points in early T-cell development. Immunol. Today 14, 547–553 (1993).
Fu, G. et al. Fine-tuning T cell receptor signaling to control T cell development. Trends Immunol. 35, 311–318 (2014).
Egawa, T. Regulation of CD4 and CD8 coreceptor expression and CD4 versus CD8 lineage decisions. Adv. Immunol. 125, 1–40 (2015).
Spidale, N. A., Wang, B. & Tisch, R. Cutting edge: Antigen-specific thymocyte feedback regulates homeostatic thymic conventional dendritic cell maturation. J. Immunol. 193, 21–25 (2014).
Klein, L., Kyewski, B., Allen, P. M. & Hogquist, K. A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat. Rev. Immunol. 14, 377–391 (2014).
Derbinski, J., Schulte, A., Kyewski, B. & Klein, L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2, 1032–1039 (2001).
Laan, M. & Peterson, P. The many faces of Aire in central tolerance. Front. Immunol. 4, 326 (2013).
Passos, G. A., Mendes-da-Cruz, D. A. & Oliveira, E. H. The thymic orchestration involving aire, miRNAs, and cell-cell interactions during the induction of central tolerance. Front. Immunol. 6, 352 (2015).
Ciofani, M. & Zuniga-Pflucker, J. C. The thymus as an inductive site for T lymphopoiesis. Annu. Rev. Cell Dev. Biol. 23, 463–493 (2007).
Petrie, H. T. & Zuniga-Pflucker, J. C. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol. 25, 649–679 (2007).
Savino, W., Mendes-da-Cruz, D. A., Silva, J. S., Dardenne, M. & Cotta-de-Almeida, V. Intrathymic T-cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 23, 305–313 (2002).
Cotta-de-Almeida, V. et al. Trypanosoma cruzi infection modulates intrathymic contents of extracellular matrix ligands and receptors and alters thymocyte migration. Eur. J. Immunol. 33, 2439–2448 (2003).
Savino, W., Mendes-Da-Cruz, D. A., Smaniotto, S., Silva-Monteiro, E. & Villa-Verde, D. M. Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix. J. Leukoc. Biol. 75, 951–961 (2004).
Michie, A. M. et al. Constitutive Notch signalling promotes CD4 CD8 thymocyte differentiation in the absence of the pre-TCR complex, by mimicking pre-TCR signals. Int. Immunol. 19, 1421–1430 (2007).
Sambandam, A. et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat. Immunol. 6, 663–670 (2005).
Varas, A. et al. Age-dependent changes in thymic macrophages and dendritic cells. Microsc. Res. Tech. 62, 501–507 (2003).
Aw, D., Silva, A. B., Maddick, M., von Zglinicki, T. & Palmer, D. B. Architectural changes in the thymus of aging mice. Aging Cell 7, 158–167 (2008).
Aw, D., Taylor-Brown, F., Cooper, K. & Palmer, D. B. Phenotypical and morphological changes in the thymic microenvironment from ageing mice. Biogerontology 10, 311–322 (2009).
Lepletier, A., Chidgey, A. P. & Savino, W. Perspectives for improvement of the thymic microenvironment through manipulation of thymic epithelial cells: a mini-review. Gerontology http://dx.doi.org/10.1159/000375160.
Qi, Q. et al. Diversity and clonal selection in the human T-cell repertoire. Proc. Natl Acad. Sci. USA 111, 13139–13144 (2014).
Gray, D. H. et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108, 3777–3785 (2006).
Griffith, A. V., Fallahi, M., Venables, T. & Petrie, H. T. Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell 11, 169–177 (2012).
Ventevogel, M. S. & Sempowski, G. D. Thymic rejuvenation and aging. Curr. Opin. Immunol. 25, 516–522 (2013).
Vacchio, M. S., Lee, J. Y. & Ashwell, J. D. Thymus-derived glucocorticoids set the thresholds for thymocyte selection by inhibiting TCR-mediated thymocyte activation. J. Immunol. 163, 1327–1333 (1999).
Charlton, H. Hypothalamic control of anterior pituitary function: a history. J. Neuroendocrinol. 20, 641–646 (2008).
de Mello-Coelho, V. et al. Growth hormone and its receptor are expressed in human thymic cells. Endocrinology 139, 3837–3842 (1998).
Ban, E. et al. Specific binding sites for growth hormone in cultured mouse thymic epithelial cells. Life Sci. 48, 2141–2148 (1991).
Hull, K. L., Thiagarajah, A. & Harvey, S. Cellular localization of growth hormone receptors/binding proteins in immune tissues. Cell Tissue Res. 286, 69–80 (1996).
Gagnerault, M. C., Postel-Vinay, M. C. & Dardenne, M. Expression of growth hormone receptors in murine lymphoid cells analyzed by flow cytofluorometry. Endocrinology 137, 1719–1726 (1996).
Taub, D. D., Murphy, W. J. & Longo, D. L. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr. Opin. Pharmacol. 10, 408–424 (2010).
Morrhaye, G. et al. Impact of growth hormone (GH) deficiency and GH replacement upon thymus function in adult patients. PLoS ONE 4, e5668 (2009).
Polgreen, L., Steiner, M., Dietz, C. A., Manivel, J. C. & Petryk, A. Thymic hyperplasia in a child treated with growth hormone. Growth Horm. IGF Res. 17, 41–46 (2007).
Kermani, H. et al. Expression of the growth hormone/insulin-like growth factor axis during Balb/c thymus ontogeny and effects of growth hormone upon ex vivo T cell differentiation. Neuroimmunomodulation 19, 137–147 (2012).
Timsit, J. et al. Growth hormone and insulin-like growth factor-I stimulate hormonal function and proliferation of thymic epithelial cells. J. Clin. Endocrinol. Metab. 75, 183–188 (1992).
Bazzoni, N. et al. Acromegaly and thymic hyperplasia: a case report. J. Endocrinol. Invest. 13, 931–935 (1990).
Smaniotto, S. et al. Combined role of extracellular matrix and chemokines on peripheral lymphocyte migration in growth hormone transgenic mice. Brain Behav. Immun. 24, 451–461 (2010).
Smaniotto, S. et al. Growth hormone modulates thymocyte development in vivo through a combined action of laminin and CXC chemokine ligand 12. Endocrinology 146, 3005–3017 (2005).
de Mello Coelho, V. et al. Functional insulin-like growth factor-1/insulin-like growth factor-1 receptor-mediated circuit in human and murine thymic epithelial cells. Neuroendocrinology 75, 139–150 (2002).
Dixit, V. D. et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 114, 57–66 (2004).
Howard, A. D. et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273, 974–977 (1996).
Dixit, V. D. et al. Ghrelin promotes thymopoiesis during aging. J. Clin. Invest. 117, 2778–2790 (2007).
Bole-Feysot, C., Goffin, V., Edery, M., Binart, N. & Kelly, P. A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19, 225–268 (1998).
DaSilva, L. et al. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol. Cell Endocrinol. 117, 131–140 (1996).
Montgomery, D. W. et al. Human thymocytes express a prolactin-like messenger ribonucleic acid and synthesize bioactive prolactin-like proteins. Endocrinology 131, 3019–3026 (1992).
Lepletier, A. et al. Trypanosoma cruzi disrupts thymic homeostasis by altering intrathymic and systemic stress-related endocrine circuitries. PLoS Negl. Trop. Dis. 7, e2470 (2013).
Dardenne, M., Kelly, P. A., Bach, J. F. & Savino, W. Identification and functional activity of prolactin receptors in thymic epithelial cells. Proc. Natl Acad. Sci. USA 88, 9700–9704 (1991).
Carreno, P. C., Jimenez, E., Sacedon, R., Vicente, A. & Zapata, A. G. Prolactin stimulates maturation and function of rat thymic dendritic cells. J. Neuroimmunol. 153, 83–90 (2004).
Carreno, P. C., Sacedon, R., Jimenez, E., Vicente, A. & Zapata, A. G. Prolactin affects both survival and differentiation of T-cell progenitors. J. Neuroimmunol. 160, 135–145 (2005).
Gagnerault, M. C., Touraine, P., Savino, W., Kelly, P. A. & Dardenne, M. Expression of prolactin receptors in murine lymphoid cells in normal and autoimmune situations. J. Immunol. 150, 5673–5681 (1993).
Dardenne, M., de Moraes Mdo, C., Kelly, P. A. & Gagnerault, M. C. Prolactin receptor expression in human hematopoietic tissues analyzed by flow cytofluorometry. Endocrinology 134, 2108–2114 (1994).
Feng, J. C., Loh, T. T. & Sheng, H. P. Lactation increases prolactin receptor expression in spleen and thymus of rats. Life Sci. 63, 111–119 (1998).
Foster, M., Montecino-Rodriguez, E., Clark, R. & Dorshkind, K. Regulation of B and T cell development by anterior pituitary hormones. Cell. Mol. Life Sci. 54, 1076–1082 (1998).
Horseman, N. D. et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16, 6926–6935 (1997).
Bouchard, B., Ormandy, C. J., Di Santo, J. P. & Kelly, P. A. Immune system development and function in prolactin receptor-deficient mice. J. Immunol. 163, 576–582 (1999).
Krishnan, N., Thellin, O., Buckley, D. J., Horseman, N. D. & Buckley, A. R. Prolactin suppresses glucocorticoid-induced thymocyte apoptosis in vivo. Endocrinology 144, 2102–2110 (2003).
Melis, M. R., Mauri, A. & Argiolas, A. Opposite changes in the content of oxytocin- and vasopressin-like immunoreactive peptides in the rat thymus during aging. Regul. Pept. 59, 335–340 (1995).
Hansenne, I. et al. Ontogenesis and functional aspects of oxytocin and vasopressin gene expression in the thymus network. J. Neuroimmunol. 158, 67–75 (2005).
Elands, J., Resink, A. & De Kloet, E. R. Neurohypophyseal hormone receptors in the rat thymus, spleen, and lymphocytes. Endocrinology 126, 2703–2710 (1990).
Hansenne, I. et al. Neurohypophysial receptor gene expression by thymic T cell subsets and thymic T cell lymphoma cell lines. Clin. Dev. Immunol. 11, 45–51 (2004).
da Silva, S. V. et al. Increased leptin response and inhibition of apoptosis in thymocytes of young rats offspring from protein deprived dams during lactation. PLoS ONE 8, e64220 (2013).
Dardenne, M., Savino, W., Gastinel, L. N., Nabarra, B. & Bach, J. F. Thymic dysfunction in the mutant diabetic (db/db) mouse. J. Immunol. 130, 1195–1199 (1983).
Gruver, A. L., Ventevogel, M. S. & Sempowski, G. D. Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution. J. Endocrinol. 203, 75–85 (2009).
Hick, R. W., Gruver, A. L., Ventevogel, M. S., Haynes, B. F. & Sempowski, G. D. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J. Immunol. 177, 169–176 (2006).
Lee, J. H. et al. Ghrelin augments murine T-cell proliferation by activation of the phosphatidylinositol-3-kinase, extracellular signal-regulated kinase and protein kinase C signaling pathways. FEBS Lett. 588, 4708–4719 (2014).
Chen, Y. K. et al. The frequency and spectrum of thymus 2-[fluorine-18] fluoro-2-deoxy-D-glucose uptake patterns in hyperthyroidism patients. Acad. Radiol. 18, 1292–1297 (2011).
Villa-Verde, D. M., de Mello-Coelho, V., Farias-de-Oliveira, D. A., Dardenne, M. & Savino, W. Pleiotropic influence of triiodothyronine on thymus physiology. Endocrinology 133, 867–875 (1993).
Ribeiro-Carvalho, M. M., Farias-de-Oliveira, D. A., Villa-Verde, D. M. & Savino, W. Triiodothyronine modulates extracellular matrix-mediated interactions between thymocytes and thymic microenvironmental cells. Neuroimmunomodulation 10, 142–152 (2002).
Ribeiro-Carvalho, M. M. et al. Triiodothyronine modulates thymocyte migration. Scand. J. Immunol. 66, 17–25 (2007).
Villa-Verde, D. M. et al. Identification of nuclear triiodothyronine receptors in the thymic epithelium. Endocrinology 131, 1313–1320 (1992).
Batanero, E. et al. The neural and neuro-endocrine component of the human thymus. II. Hormone immunoreactivity. Brain Behav. Immun. 6, 249–264 (1992).
van der Weerd, K. et al. Thyrotropin acts as a T-cell developmental factor in mice and humans. Thyroid 24, 1051–1061 (2014).
Stefan, M. et al. Genetic-epigenetic dysregulation of thymic TSH receptor gene expression triggers thyroid autoimmunity. Proc. Natl Acad. Sci. USA 111, 12562–12567 (2014).
Montagne, J. J., Ladram, A., Nicolas, P. & Bulant, M. Cloning of thyrotropin-releasing hormone precursor and receptor in rat thymus, adrenal gland, and testis. Endocrinology 140, 1054–1059 (1999).
Matre, V. et al. The human neuroendocrine thyrotropin-releasing hormone receptor promoter is activated by the haematopoietic transcription factor c-Myb. Biochem. J. 372, 851–859 (2003).
Pawlikowski, M., Zerek-Melen, G. & Winczyk, K. Thyroliberin (TRH) increases thymus cell proliferation in rats. Neuropeptides 23, 199–202 (1992).
Kawashima, I. et al. Localization of estrogen receptors and estrogen receptor-mRNA in female mouse thymus. Thymus 20, 115–121 (1992).
Nancy, P. & Berrih-Aknin, S. Differential estrogen receptor expression in autoimmune myasthenia gravis. Endocrinology 146, 2345–2353 (2005).
Viselli, S. M., Olsen, N. J., Shults, K., Steizer, G. & Kovacs, W. J. Immunochemical and flow cytometric analysis of androgen receptor expression in thymocytes. Mol. Cell Endocrinol. 109, 19–26 (1995).
Olsen, N. J., Watson, M. B., Henderson, G. S. & Kovacs, W. J. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology 129, 2471–2476 (1991).
Yellayi, S. et al. Normal development of thymus in male and female mice requires estrogen/estrogen receptor-α signaling pathway. Endocrine 12, 207–213 (2000).
Staples, J. E. et al. Estrogen receptor α is necessary in thymic development and estradiol-induced thymic alterations. J. Immunol. 163, 4168–4174 (1999).
Ishibashi, H. et al. Estrogen inhibits cell proliferation through in situ production in human thymoma. Clin. Cancer Res. 11, 6495–6504 (2005).
Utsuyama, M. & Hirokawa, K. Hypertrophy of the thymus and restoration of immune functions in mice and rats by gonadectomy. Mech. Ageing Dev. 47, 175–185 (1989).
Azad, N., Emanuele, N. V., Halloran, M. M., Tentler, J. & Kelley, M. R. Presence of luteinizing hormone-releasing hormone (LHRH) mRNA in rat spleen lymphocytes. Endocrinology 128, 1679–1681 (1991).
Marchetti, B. et al. Luteinizing hormone-releasing hormone (LHRH) agonist restoration of age-associated decline of thymus weight, thymic LHRH receptors, and thymocyte proliferative capacity. Endocrinology 125, 1037–1045 (1989).
Cohen, J. J. Glucocorticoid-induced apoptosis in the thymus. Semin. Immunol. 4, 363–369 (1992).
Berki, T., Palinkas, L., Boldizsar, F. & Nemeth, P. Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations. Int. Immunol. 14, 463–469 (2002).
Mittelstadt, P. R., Monteiro, J. P. & Ashwell, J. D. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J. Clin. Invest. 122, 2384–2394
Pálinkás, L. et al. Developmental shift in TcR-mediated rescue of thymocytes from glucocorticoid-induced apoptosis. Immunobiology 213, 39–50 (2008).
Purton, J. F., Boyd, R. L., Cole, T. J. & Godfrey, D. I. Intrathymic T cell development and selection proceeds normally in the absence of glucocorticoid receptor signaling. Immunity 13, 179–186 (2000).
Tsawdaroglou, N. G., Govindan, M. V., Schmid, W. & Sekeris, C. E. Dexamethasone-binding proteins in cytosol and nucleus of rat thymocytes. Purification of three receptor proteins. Eur. J. Biochem. 114, 305–313 (1981).
McGimsey, W. C., Cidlowski, J. A., Stumpf, W. E. & Sar, M. Immunocytochemical localization of the glucocorticoid receptor in rat brain, pituitary, liver, and thymus with two new polyclonal antipeptide antibodies. Endocrinology 129, 3064–3072 (1991).
Ratman, D. et al. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol. Cell. Endocrinol. 380, 41–54 (2013).
Carlberg, C. & Seuter, S. Dynamics of nuclear receptor target gene regulation. Chromosoma 119, 479–484 (2010).
Stavreva, D. A. et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat. Cell Biol. 11, 1093–1102 (2009).
Ranelletti, F. O. et al. Glucocorticoid receptors and corticosensitivity of human thymocytes at discrete stages of intrathymic differentiation. J. Immunol. 138, 440–445 (1987).
Dardenne, M., Itoh, T. & Homo-Delarche, F. Presence of glucocorticoid receptors in cultured thymic epithelial cells. Cell. Immunol. 100, 112–118 (1986).
Kino, T., Su, Y. A. & Chrousos, G. P. Human glucocorticoid receptor isoform β: recent understanding of its potential implications in physiology and pathophysiology. Cell. Mol. Life Sci. 66, 3435–3448 (2009).
Oakley, R. H., Webster, J. C., Sar, M., Parker, C. R. Jr & Cidlowski, J. A. Expression and subcellular distribution of the β-isoform of the human glucocorticoid receptor. Endocrinology 138, 5028–5038 (1997).
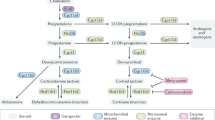
Lechner, O. et al. Glucocorticoid production in the murine thymus. Eur. J. Immunol. 30, 337–346 (2000).
Vacchio, M. S., Papadopoulos, V. & Ashwell, J. D. Steroid production in the thymus: implications for thymocyte selection. J. Exp. Med. 179, 1835–1846 (1994).
Qiao, S., Chen, L., Okret, S. & Jondal, M. Age-related synthesis of glucocorticoids in thymocytes. Exp. Cell Res. 314, 3027–3035 (2008).
Lacaze-Masmonteil, T., de Keyzer, Y., Luton, J. P., Kahn, A. & Bertagna, X. Characterization of proopiomelanocortin transcripts in human nonpituitary tissues. Proc. Natl Acad. Sci. USA 84, 7261–7265 (1987).
Jessop, D. S., Renshaw, D., Lightman, S. L. & Harbuz, M. S. Changes in ACTH and β-endorphin immunoreactivity in immune tissues during a chronic inflammatory stress are not correlated with changes in corticotropin-releasing hormone and arginine vasopressin. J. Neuroimmunol. 60, 29–35 (1995).
Talaber, G., Tuckermann, J. P. & Okret, S. ACTH controls thymocyte homeostasis independent of glucocorticoids. FASEB J. 29, 2526–2534 (2015).
Aird, F., Clevenger, C. V., Prystowsky, M. B. & Redei, E. Corticotropin-releasing factor mRNA in rat thymus and spleen. Proc. Natl Acad. Sci. USA 90, 7104–7108 (1993).
Savino, W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2, e62 (2006).
Mendes-da-Cruz, D. A., de Meis, J., Cotta-de-Almeida, V. & Savino, W. Experimental Trypanosoma cruzi infection alters the shaping of the central and peripheral T-cell repertoire. Microbes Infect. 5, 825–832 (2003).
Morrot, A. et al. Chagasic thymic atrophy does not affect negative selection but results in the export of activated CD4+CD8+ T cells in severe forms of human disease. PLoS Negl. Trop. Dis. 5, e1268 (2011).
Mendes-da-Cruz, D. A., Silva, J. S., Cotta-de-Almeida, V. & Savino, W. Altered thymocyte migration during experimental acute Trypanosoma cruzi infection: combined role of fibronectin and the chemokines CXCL12 and CCL4. Eur. J. Immunol. 36, 1486–1493 (2006).
Perez, A. R. et al. Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav. Immun. 21, 890–900 (2007).
Roggero, E. et al. Endogenous glucocorticoids cause thymus atrophy but are protective during acute Trypanosoma cruzi infection. J. Endocrinol. 190, 495–503 (2006).
Correa-de-Santana, E. et al. Hypothalamus-pituitary-adrenal axis during Trypanosoma cruzi acute infection in mice. J. Neuroimmunol. 173, 12–22 (2006).
Salas, M. A., Evans, S. W., Levell, M. J. & Whicher, J. T. Interleukin-6 and ACTH act synergistically to stimulate the release of corticosterone from adrenal gland cells. Clin. Exp. Immunol. 79, 470–473 (1990).
Correa-de-Santana, E. et al. Modulation of growth hormone and prolactin secretion in Trypanosoma cruzi-infected mammosomatotrophic cells. Neuroimmunomodulation 16, 208–212 (2009).
Lechner, J., Welte, T. & Doppler, W. Mechanism of interaction between the glucocorticoid receptor and Stat5: role of DNA-binding. Immunobiology 198, 112–123 (1997).
Gruver, A. L., Hudson, L. L. & Sempowski, G. D. Immunosenescence of ageing. J. Pathol. 211, 144–156 (2007).
Kovaiou, R. D. et al. Age-related differences in phenotype and function of CD4+ T cells are due to a phenotypic shift from naive to memory effector CD4+ T cells. Int. Immunol. 17, 1359–1366 (2005).
Chiu, B. C., Martin, B. E., Stolberg, V. R. & Chensue, S. W. Cutting edge: Central memory CD8 T cells in aged mice are virtual memory cells. J. Immunol. 191, 5793–5796 (2013).
Grody, W. W., Fligiel, S. & Naeim, F. Thymus involution in the acquired immunodeficiency syndrome. Am. J. Clin. Pathol. 84, 85–95 (1985).
Nezelof, C. Thymic pathology in primary and secondary immunodeficiencies. Histopathology 21, 499–511 (1992).
Hermann, M. & Berger, P. Hormonal changes in aging men: a therapeutic indication? Exp. Gerontol. 36, 1075–1082 (2001).
Savino, W., Postel-Vinay, M. C., Smaniotto, S. & Dardenne, M. The thymus gland: a target organ for growth hormone. Scand. J. Immunol. 55, 442–452 (2002).
Kelley, K. W. et al. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc. Natl Acad. Sci. USA 83, 5663–5667 (1986).
Savino, W., Smaniotto, S., Binart, N., Postel-Vinay, M. C. & Dardenne, M. In vivo effects of growth hormone on thymic cells. Ann. N. Y. Acad. Sci. 992, 179–185 (2003).
Rosenfeld, R. G. et al. Growth hormone insensitivity resulting from post-GH receptor defects. Growth Horm. IGF Res. 14 (Suppl. A), S35–S38 (2004).
Pugliese-Pires, P. N. et al. A novel STAT5B mutation causing GH insensitivity syndrome associated with hyperprolactinemia and immune dysfunction in two male siblings. Eur. J. Endocrinol. 163, 349–355 (2010).
Dardenne, M., Smaniotto, S., de Mello-Coelho, V., Villa-Verde, D. M. & Savino, W. Growth hormone modulates migration of developing T cells. Ann. N. Y. Acad. Sci. 1153, 1–5 (2009).
French, R. A. et al. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology 143, 690–699 (2002).
Smaniotto, S., Ribeiro-Carvalho, M. M., Dardenne, M., Savino, W. & de Mello-Coelho, V. Growth hormone stimulates the selective trafficking of thymic CD4+CD8- emigrants to peripheral lymphoid organs. Neuroimmunomodulation 11, 299–306 (2004).
Montecino-Rodriguez, E., Clark, R. & Dorshkind, K. Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology 139, 4120–4126 (1998).
Chu, Y. W. et al. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood 112, 2836–2846 (2008).
Youm, Y. H. et al. Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J. Biol. Chem. 284, 7068–7077 (2009).
Greenwood, P. L. & Bell, A. W. Consequences of intra-uterine growth retardation for postnatal growth, metabolism and pathophysiology. Reprod. Suppl. 61, 195–206 (2003).
Barr, I. G. et al. Dihydrotestosterone and estradiol deplete corticosensitive thymocytes lacking in receptors for these hormones. J. Immunol. 128, 2825–2828 (1982).
Kendall, M. D. et al. Reversal of ageing changes in the thymus of rats by chemical or surgical castration. Cell Tissue Res. 261, 555–564 (1990).
Heng, T. S. et al. Effects of castration on thymocyte development in two different models of thymic involution. J. Immunol. 175, 2982–2993 (2005).
Greenstein, B. D., Fitzpatrick, F. T., Kendall, M. D. & Wheeler, M. J. Regeneration of the thymus in old male rats treated with a stable analogue of LHRH. J. Endocrinol. 112, 345–350 (1987).
Velardi, E. et al. Sex steroid blockade enhances thymopoiesis by modulating notch signaling. J. Exp. Med. 211, 2341–2349 (2014).
Mattsson, C. & Olsson, T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr. Med. Chem. 14, 2918–2924 (2007).
Zhao, H., Tian, Z., Hao, J. & Chen, B. Extragonadal aromatization increases with time after ovariectomy in rats. Reprod. Biol. Endocrinol. 3, 6 (2005).
Limonta, P. et al. GnRH receptors in cancer: from cell biology to novel targeted therapeutic strategies. Endocr. Rev. 33, 784–811 (2012).
Savino, W. et al. Thymic epithelium in AIDS. An immunohistologic study. Am. J. Pathol. 122, 302–307 (1986).
Napolitano, L. A. et al. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS 16, 1103–1111 (2002).
Tesselaar, K. & Miedema, F. Growth hormone resurrects adult human thymus during HIV-1 infection. J. Clin. Invest. 118, 844–847 (2008).
Haynes, B. F. HIV infection and the dynamic interplay between the thymus and the peripheral T cell pool. Clin. Immunol. 92, 3–5 (1999).
Napolitano, L. A. et al. Growth hormone enhances thymic function in HIV-1-infected adults. J. Clin. Invest. 118, 1085–1098 (2008).
Herasimtschuk, A. A. et al. Low-dose growth hormone for 40 weeks induces HIV-1-specific T cell responses in patients on effective combination anti-retroviral therapy. Clin. Exp. Immunol. 173, 444–453 (2013).
Melis, M. R., Stancampiano, R. & Argiolas, A. Oxytocin- and vasopressin-like immunoreactivity in the rat thymus: characterization and possible involvement in the immune response. Regul. Pept. 45, 269–272 (1993).
Johnson, E. W., Hughes, T. K. Jr & Smith, E. M. ACTH receptor distribution and modulation among murine mononuclear leukocyte populations. J. Biol. Regul. Homeost. Agents 15, 156–162 (2001).
Kim, S. Y. et al. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem. Biophys. Res. Commun. 394, 562–568 (2010).
Brtko, J. & Knopp, J. Rat thymus: demonstration of specific thyroxine receptors in nuclear extract. Endocrinol. Exp. 17, 3–9 (1983).
Howard, J. K. et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Invest. 104, 1051–1059 (1999).
Hareramadas, B. & Rai, U. Mechanism of androgen-induced thymic atrophy in the wall lizard, Hemidactylus flaviviridis: an in vitro study. Gen. Comp. Endocrinol. 144, 10–19 (2005).
De Mello-Coelho, V., Savino, W., Postel-Vinay, M. C. & Dardenne, M. Role of prolactin and growth hormone on thymus physiology. Dev. Immunol. 6, 317–323 (1998).
Olsen, N. J., Olson, G., Viselli, S. M., Gu, X. & Kovacs, W. J. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 142, 1278–1283 (2001).
Sacedon, R. et al. Partial blockade of T-cell differentiation during ontogeny and marked alterations of the thymic microenvironment in transgenic mice with impaired glucocorticoid receptor function. J. Neuroimmunol. 98, 157–167 (1999).
Jondal, M., Pazirandeh, A. & Okret, S. Different roles for glucocorticoids in thymocyte homeostasis? Trends Immunol. 25, 595–600 (2004).
Lin, B., Kinoshita, Y., Hato, F. & Tsuji, Y. Enhancement of thymic lymphocyte proliferation by the culture supernatant of thymus epithelial cells stimulated by prolactin. Cell. Mol. Biol. (Noisy-le-grand) 43, 361–367 (1997).
Pazirandeh, A., Xue, Y., Prestegaard, T., Jondal, M. & Okret, S. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J. 16, 727–729 (2002).
Mihara, S. et al. Effects of thyroid hormones on apoptotic cell death of human lymphocytes. J. Clin. Endocrinol. Metab. 84, 1378–1385 (1999).
Olsen, N. J., Viselli, S. M., Fan, J. & Kovacs, W. J. Androgens accelerate thymocyte apoptosis. Endocrinology 139, 748–752 (1998).
Belloni, A. S. et al. Effect of ghrelin on the apoptotic deletion rate of different types of cells cultured in vitro. Int. J. Mol. Med. 14, 165–167 (2004).
Alpdogan, O. et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation 75, 1977–1983 (2003).
Montgomery, D. W., Krumenacker, J. S. & Buckley, A. R. Prolactin stimulates phosphorylation of the human T-cell antigen receptor complex and ZAP-70 tyrosine kinase: a potential mechanism for its immunomodulation. Endocrinology 139, 811–814 (1998).
Tsuji, Y., Kinoshita, Y., Hato, F., Tominaga, K. & Yoshida, K. The in vitro proliferation of thymus epithelial cells stimulated with growth hormone and insulin-like growth factor-I. Cell. Mol. Biol. (Noisy-le-grand) 40, 1135–1142 (1994).
Sakabe, K., Kawashima, I., Urano, R., Seiki, K. & Itoh, T. Effects of sex steroids on the proliferation of thymic epithelial cells in a culture model: a role of protein kinase C. Immunol. Cell Biol. 72, 193–199 (1994).
Talaber, G. et al. Wnt-4 protects thymic epithelial cells against dexamethasone-induced senescence. Rejuvenation Res. 14, 241–248 (2011).
Dardenne, M. et al. Thymic hormone-containing cells. VII. Adrenals and gonads control the in vivo secretion of thymulin and its plasmatic inhibitor. J. Immunol. 136, 1303–1308 (1986).
Coura, J. R. & Vinas, P. A. Chagas disease: a new worldwide challenge. Nature 465, S6–S7 (2010).
Perez, A. R. et al. Immunoneuroendocrine alterations in patients with progressive forms of chronic Chagas disease. J. Neuroimmunol. 235, 84–90 (2011).
Savino, W., Leite-de-Moraes, M. C., Hontebeyrie-Joskowicz, M. & Dardenne, M. Studies on the thymus in Chagas' disease. I. Changes in the thymic microenvironment in mice acutely infected with Trypanosoma cruzi. Eur. J. Immunol. 19, 1727–1733 (1989).
Vilar-Pereira, G. et al. Trypanosoma cruzi-induced depressive-like behavior is independent of meningoencephalitis but responsive to parasiticide and TNF-targeted therapeutic interventions. Brain Behav. Immun. 26, 1136–1149 (2012).
Perez, A. R., Bottasso, O. & Savino, W. The impact of infectious diseases upon neuroendocrine circuits. Neuroimmunomodulation 16, 96–105 (2009).
Acknowledgements
Work in the authors' laboratories was supported with grants from CNPq, Capes, Faperj and Fiocruz (Brazil), FOCEM (Mercosur countries) and CNRS (France). The conjoint work was developed in the framework of the Fiocruz–CNRS International Associated Laboratory of Immunology and Immunopathology.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, wrote, reviewed and edited the manuscript before submission. D.A.M.-d.-C., A.L. and W.S. made substantial contribution to discussion of the content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Savino, W., Mendes-da-Cruz, D., Lepletier, A. et al. Hormonal control of T-cell development in health and disease. Nat Rev Endocrinol 12, 77–89 (2016). https://doi.org/10.1038/nrendo.2015.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.168
This article is cited by
-
Effect of Glycyrrhetic Acid Derivatives on Regulation of Thymocyte Volume
Bulletin of Experimental Biology and Medicine (2023)
-
IGF-1 increases survival of CD4+ lineage in a 2D model of thymocyte/thymic stromal cell co-culture
In Vitro Cellular & Developmental Biology - Animal (2022)
-
Early-life oxytocin attenuates the social deficits induced by caesarean-section delivery in the mouse
Neuropsychopharmacology (2021)
-
CGRP-Mediated Prolactin Upregulation: a Possible Pathomechanism in IgG4-Related Disease
Inflammation (2021)
-
CXCL12-driven thymocyte migration is increased by thymic epithelial cells treated with prolactin in vitro
Journal of Biosciences (2021)