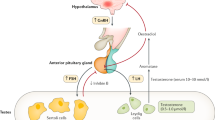
Abstract
Findings in the past few years have advanced understanding of the crosstalk between testis and bone and could contribute to defining an improved clinical approach to the biochemical diagnosis and therapeutic management of hypogonadism and male osteoporosis. This Review focuses on the Leydig cells of the testis. Other than being responsible for steroidogenesis and production of testosterone, the function of these cells is fundamental to bone health in at least two other ways: Leydig cells produce insulin-like 3 (INSL3), which has a role in osteoblast function, and they contribute to 25-hydroxylation of vitamin D. Impairment of testicular function leads to low levels of testosterone, INSL3 and 25-hydroxyvitamin D and consequently to an increased risk of osteopenia and osteoporosis.
Key Points
-
Hypogonadism is a common secondary cause of male osteoporosis; however, men with mild testicular dysfunction are also at increased risk of osteopenia and osteoporosis
-
Knowledge of the bidirectional crosstalk between testis and bone obtained in the past few years could inform the clinical approach to the diagnosis and therapeutic management of hypogonadism and male osteoporosis
-
The testis might also contribute to bone health in a testosterone-independent manner by production of insulin-like 3 (INSL3) and by contributing to the 25-hydroxylation of vitamin D
-
INSL3 and 25-hydroxyvitamin D are sensitive markers of Leydig cell function in subclinical hypogonadism, when their levels are reduced and might contribute to low bone mass despite normal testosterone levels
-
Testosterone replacement therapy alone in men with hypogonadism does not completely restore bone mass, which suggests that alternative therapeutic approaches should be considered in future studies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Karsenty, G. The mutual dependence between bone and gonads. J. Endocrinol. 213, 107–114 (2012).
Ebeling, P. R. Clinical practice. Osteoporosis in men. N. Engl. J. Med. 358, 1474–1482 (2008).
Bhasin, S. et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 95, 2536–2559 (2010).
Watts, N. B. et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 97, 1802–1822 (2012).
Ferlin, A. et al. Mutations in the insulin-like factor 3 receptor are associated with osteoporosis. J. Bone Miner. Res. 23, 683–693 (2008).
Ferlin, A., Perilli, L., Gianesello, L., Taglialavoro, G. & Foresta, C. Profiling insulin like factor 3 (INSL3) signaling in human osteoblasts. PLoS ONE 6, e29733 (2011).
Foresta. C. et al. A novel circulating hormone of testis origin in humans. J. Clin. Endocrinol. Metab. 89, 5952–5958 (2004).
Bay, K. et al. Insulin-like factor 3 serum levels in 135 normal men and 85 men with testicular disorders: relationship to the luteinizing hormone-testosterone axis. J. Clin. Endocrinol. Metab. 90, 3410–3418 (2005).
Sadeghian, H., Anand-Ivell, R., Balvers, M., Relan, V. & Ivell, R. Constitutive regulation of the Insl3 gene in rat Leydig cells. Mol. Cell. Endocrinol. 241, 10–20 (2005).
Anand-Ivell, R. et al. Peripheral INSL3 concentrations decline with age in a large population of Australian men. Int. J. Androl. 29, 618–626 (2006).
Bay, K., Matthiesson, K. L., McLachlan, R. I. & Andersson, A. M. The effects of gonadotropins suppression and selective replacement on insulin-like factor 3 secretion in normal adult men. J. Clin. Endocrinol. Metab. 91, 1108–1111 (2006).
Ferlin, A. et al. Changes in serum insulin-like factor 3 during normal male puberty. J. Clin. Endocrinol. Metab. 91, 3426–3431 (2006).
Foresta, C. et al. Insulin-like factor 3 as a marker of testicular function in obese men. Clin. Endocrinol. (Oxf) 71, 722–726 (2009).
Ivell, R. & Anand-Ivell, R. Biological role and clinical significance of insulin-like peptide 3. Curr. Opin. Endocrinol. Diabetes Obes. 18, 210–216 (2011).
Foresta, C. et al. Bone mineral density and testicular failure: evidence for a role of vitamin D25 hydroxylase in human testis. J. Clin. Endocrinol. Metab. 96, E646–E652 (2011).
Blomberg Jensen, M. et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 25, 1303–1311 (2010).
Lee, D. M. et al. Association of hypogonadism with vitamin D status: the European Male Ageing Study. Eur. J. Endocrinol. 166, 77–85 (2012).
Wehr, E., Pilz, S., Boehm, B. O., März, W. & Obermayer-Pietsch, B. Association of vitamin D status with serum androgen levels in men. Clin. Endocrinol. (Oxf.) 73, 243–248 (2010).
Nimptsch, K., Platz, E. A., Willett, W. C. & Giovannucci, E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin. Endocrinol. (Oxf.) 77, 106–112 (2012).
Oury, F. et al. Endocrine regulation of male fertility by the skeleton. Cell. 144, 796–809 (2011).
Pi, M. & Quarles, L. D. Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology 153, 2062–2069 (2012).
Pi, M. et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 3, e3858 (2008).
Hannemann, A. et al. Osteocalcin is associated with testosterone in the general population and selected patients with bone disorders. Andrology 1, 469–474 (2013).
Pi, M., Parrill, A. L. & Quarles, L. D. GPRC6A mediates the non-genomic effects of steroids. J. Biol. Chem. 285, 39953–39964 (2010).
Nakamura, T. et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007).
Venken, K., Callewaert, F., Boonen, S. & Vanderschueren, D. Sex hormones, their receptors and bone health. Osteoporos. Int. 19, 1517–1525 (2008).
Callewaert, F., Sinnesael, M., Gielen, E., Boonen, S. & Vanderschueren, D. Skeletal sexual dimorphism: relative contribution of sex steroids. J. Endocrinol. 207, 127–134 (2010).
Vanderschueren, D. et al. Androgens and bone. Endocr. Rev. 25, 389–425 (2004).
Callewaert, F., Boonen, S. & Vanderschueren, D. Sex steroids and the male skeleton: a tale of two hormones. Trends Endocrinol. Metab. 21, 89–99 (2010).
Marcus, R. et al. The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome. J. Clin. Endocrinol. Metab. 85, 1032–1037 (2000).
Khosla, S., Melton L. J. 3rd, Atkinson, E. J. & O'Fallon, W. M. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J. Clin. Endocrinol. Metab. 86, 3555–3561 (2001).
Rochira, V. et al. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidences of a priming effect of estrogen for sex steroids action on bone. Bone 40, 1662–1668 (2007).
Callewaert, F. et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-α. FASEB J. 23, 232–240 (2009).
Mellström, D. et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J. Bone Miner. Res. 23, 1552–1560 (2008).
Amin, S. et al. Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am. J. Med. 119, 426–433 (2006).
Goderie-Plomp, H. W. et al. Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. J. Clin. Endocrinol. Metab. 89, 3261–3269 (2004).
Falahati-Nini, A. et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Invest. 106, 1553–1560 (2000).
Francis, R. M. The effects of testosterone on osteoporosis in men. Clin. Endocrinol. (Oxf). 50, 411–414 (1999).
Clarke, B. L. & Khosla, S. Androgens and bone. Steroids 74, 296–305 (2009).
Giannetta, E., Gianfrilli, D., Barbagallo, F., Isidori, A. M. & Lenzi, A. Subclinical male hypogonadism. Best Pract. Res. Clin. Endocrinol. Metab. 26, 539–550 (2012).
Foresta, C., Zuccarello, D., Garolla, A. & Ferlin, A. Role of hormones, genes, and environment in human cryptorchidism. Endocr. Rev. 29, 560–580 (2008).
Ferlin, A. et al. Genetic alterations associated with cryptorchidism. JAMA 300, 2271–2276 (2008).
Bogatcheva, N. V. et al. T222P mutation of the insulin-like 3 hormone receptor LGR8 is associated with testicular maldescent and hinders receptor expression on the cell surface membrane. Am. J. Physiol. Endocrinol. Metab. 292, E138–E144 (2007).
Overbeek, P. A. et al. A transgenic insertion causing cryptorchidism in mice. Genesis 30, 26–35 (2001).
Battault, S. et al. Vitamin D metabolism, functions and needs: from science to health claims. Eur. J. Nutr. 52, 429–441 (2013).
Zhu, J. & DeLuca, H. F. Vitamin D 25-hydroxylase—Four decades of searching, are we there yet? Arch. Biochem. Biophys. 523, 30–36 (2012).
Cheng, J. B., Motola, D. L., Mangelsdorf, D. J. & Russell, D. W. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxylase. J. Biol. Chem. 278, 38084–38093 (2003).
Cheng, J. B., Levine, M. A., Bell, N. H., Mangelsdorf, D. J. & Russell, D. W. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl Acad. Sci. USA 101, 7711–7715 (2004).
Demay, M. B. Mechanism of vitamin D receptor action. Ann. NY Acad. Sci. 1068, 204–213 (2006).
Wang, T. J. et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376, 180–188 (2010).
Bièche, I. et al. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet. Genomics 17, 731–742 (2007).
Choudhary, D., Jansson, I., Stoilov, I., Sarfarazi, M. & Schenkman, J. B. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch. Biochem. Biophys. 436, 50–61 (2005).
Foresta, C., Selice, R., Di Mambro, A. & Strapazzon, G. Testiculopathy and vitamin D insufficiency. Lancet 376, 1301 (2010).
Foresta, C. et al. Altered bone status in unilateral testicular cancer survivors: role of CYP2R1 and its luteinizing hormone-dependency. J. Endocrinol. Invest. 36, 379–384 (2013).
Blomberg Jensen, M. et al. Expression of the vitamin D receptor, 25-hydroxylases, 1α-hydroxylase and 24-hydroxylase in the human kidney and renal clear cell cancer. J. Steroid Biochem. Mol. Biol. 121, 376–382 (2010).
Blomberg Jensen, M. et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int. J. Androl. 35, 499–510 (2012).
Deb, S. & Bandiera, S. M. Regulation of cytochrome P450 1B1 expression by luteinizing hormone in mouse MA-10 and rat R2C Leydig cells: role of protein kinase A. Biol. Reprod. 85, 89–96 (2011).
Pilz, S. et al. Effect of vitamin D supplementation on testosterone levels in men. Horm. Metab. Res. 43, 223–225 (2011).
Wang, C. et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur. Urol. 55, 121–130 (2009).
Wu, F. C. et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 363, 123–135 (2010).
Ivell, R. & Anand-Ivell, R. Biology of insulin-like factor 3 in human reproduction. Hum. Reprod. Update 15, 463–476 (2009).
Adham, I. M., Burkhardt, E., Benahmed, M. & Engel, W. Cloning of a cDNA for a novel insulin-like peptide of the testicular Leydig cells. J. Biol. Chem. 268, 26668–26672 (1993).
Kong, R. C., Shilling, P. J., Lobb, D. K., Goolet, P. R. & Bathgate, R. A. Membrane receptors: structure and function of the relaxin family peptide receptors. Mol. Cell. Endocrinol. 320, 1–15 (2010).
Nef, S. & Parada, L. F. Cryptorchidism in mice mutant for Insl3. Nat. Genet. 22, 295–299 (1999).
Zimmermann, S. et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 13, 681–691 (1999).
Gorlov, I. P. et al. Mutations of the GREAT gene cause cryptorchidism. Hum. Mol. Genet. 11, 2309–2318 (2002).
Bay, K. et al. Insulin-like factor 3 levels in cord blood and serum from children: effects of age, postnatal hypothalamic–pituitary–gonadal axis activation, and cryptorchidism. J. Clin. Endocrinol. Metab. 92, 4020–4027 (2007).
Author information
Authors and Affiliations
Contributions
A. Ferlin, R. Selice and U. Carraro researched data for the article, provided a substantial contribution to a discussion of the content, wrote the article, and reviewed and/or edited the manuscript before submission. C. Foresta provided a substantial contribution to a discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ferlin, A., Selice, R., Carraro, U. et al. Testicular function and bone metabolism—beyond testosterone. Nat Rev Endocrinol 9, 548–554 (2013). https://doi.org/10.1038/nrendo.2013.135
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.135
This article is cited by
-
Androgen insensitivity syndrome: a review
Journal of Endocrinological Investigation (2023)
-
Adult- and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE)
Journal of Endocrinological Investigation (2022)
-
Testosterone supplementation and bone parameters: a systematic review and meta-analysis study
Journal of Endocrinological Investigation (2022)
-
Impact of hypogonadism on bone mineral density and vertebral fractures in HIV-infected men
Journal of Endocrinological Investigation (2022)
-
Ovarian 25OH-vitamin D production in young women affected by polycystic ovary syndrome
Journal of Endocrinological Investigation (2020)