Abstract
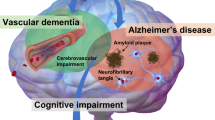
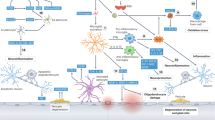
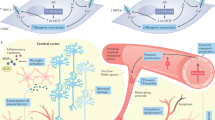
The term vascular cognitive impairment (VCI) was introduced around the start of the new millennium and refers to the contribution of vascular pathology to any severity of cognitive impairment, ranging from subjective cognitive decline and mild cognitive impairment to dementia. Although vascular pathology is common in elderly individuals with cognitive decline, pure vascular dementia (that is, dementia caused solely by vascular pathology) is uncommon. Indeed, most patients with vascular dementia also have other types of pathology, the most common of which is Alzheimer disease (specifically, the diffuse accumulation of amyloid-β plaques and neurofibrillary tangles composed of tau). At present, the main treatment for VCI is prevention by treating vascular diseases and other risk factors for VCI, such as hypertension and diabetes mellitus. Despite the current paucity of disease-modifying pharmacological treatments, we foresee that eventually, we might be able to target specific brain diseases to prevent cognitive decline and dementia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hachinski, V. C. & Bowler, J. V. Vascular dementia. Neurology 43, 2159–2161 (1993).
Hachinski, V. Vascular dementia: a radical redefinition. Dementia 5, 130–132 (1994).
O'Brien, J. T. et al. Vascular cognitive impairment. Lancet Neurol. 2, 89–98 (2003).
Gorelick, P. B. et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713 (2011). This paper provides an overview of the definition, scope and knowledge of VCI.
Román, G. C. et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260 (1993).
Hejl, A. Potentially reversible conditions in 1000 consecutive memory clinic patients. J. Neurol. Neurosurg. Psychiatry 73, 390–394 (2002).
Barker, W. W. et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 16, 203–212 (2002).
Schneider, J. A., Arvanitakis, Z., Bang, W. & Bennett, D. A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204 (2007).
Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study(MRC CFAS). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 357, 169–175 (2001).
Sachdev, P. et al. Diagnostic criteria for vascular cognitive disorders. Alzheimer Dis. Assoc. Disord. 28, 206–218 (2014).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, 2013).
Skrobot, O. A. et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 13, 624–633 (2016).
Skoog, I. in Principles and Practice of Geriatric Psychiatry 3rd edn (eds Abou-Saleh, M. T., Katona, C. L. E. & Kumar, A. ) (Wiley-Blackwell, 2011).
Goodman, R. A. et al. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 13, 28–37 (2017).
Toledo, J. B. et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 136, 2697–2706 (2013).
Prince, M. et al. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e2 (2013).
United Nations. World Population Prospects (United Nations, New York, 2013).
Andersson, M. et al. A population-based study on dementia and stroke in 97 year olds. Age Ageing 41, 529–533 (2012).
von Strauss, E., Viitanen, M., De Ronchi, D., Winblad, B. & Fratiglioni, L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch. Neurol. 56, 587–592 (1999).
Corraini, P. et al. Long-term risk of dementia among survivors of ischemic or hemorrhagic stroke. Stroke 48, 180–186 (2016).
Savva, G. M. et al. Age, neuropathology, and dementia. N. Engl. J. Med. 360, 2302–2309 (2009).
Kua, E. H. et al. The natural history of dementia. Psychogeriatrics 14, 196–201 (2014).
Staekenborg, S. S., Pijnenburg, Y. A. L., Lemstra, A. W., Scheltens, P. & van de Flier, W. M. Dementia and rapid mortality: who is at risk? J. Alzheimers Dis. 53, 135–142 (2016).
Kim, J. H. et al. Survival in subcortical vascular dementia: predictors and comparison to probable Alzheimer's disease in a tertiary memory clinic population. Dement. Geriatr. Cogn. Disord. 40, 210–221 (2015).
Skoog, I. et al. Decreasing prevalence of dementia in 85-year olds examined 22 years apart: the influence of education and stroke. Sci. Rep. 7, 6136 (2017).
Khatib, R. et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet 387, 61–69 (2016).
Rizzi, L., Rosset, I. & Roriz-Cruz, M. Global epidemiology of dementia: Alzheimer's and vascular types. Biomed. Res. Int. 2014, 908915 (2014).
Ohara, T. et al. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 88, 1925–1932 (2017).
Zhang, Y. et al. Prevalence of dementia and major dementia subtypes in the Chinese populations: a meta-analysis of dementia prevalence surveys, 1980–2010. J. Clin. Neurosci. 19, 1333–1337 (2012).
Satizabal, C. L. et al. Incidence of dementia over three decades in the Framingham Heart Study. N. Engl. J. Med. 374, 523–532 (2016). This study demonstrates a decreased incidence of dementia between the late 1970s and early 2010s. This study also demonstrates a reduction in the risk of dementia in relation to stroke and vascular disorders, such as atrial fibrillation and heart failure, during the study period, suggesting that better treatment of stroke and vascular risk factors can influence the risk of dementia.
Wu, Y.-T. et al. The changing prevalence and incidence of dementia over time? Current evidence. Nat. Rev. Neurol. 13, 327–339 (2017).
Wu, Y.-T. et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 15, 116–124 (2016).
Feigin, V. L. et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383, 245–255 (2014).
Sacco, R. L. & Dong, C. Declining stroke incidence and improving survival in US communities. JAMA 312, 237 (2014).
Zhi, X. et al. Prevalence of cardiovascular disorders and risk factors in two 75-year-old birth cohorts examined in 1976–1977 and 2005–2006. Aging Clin. Exp. Res. 25, 377–383 (2013).
Lindén, T., Skoog, I., Fagerberg, B., Steen, B. & Blomstrand, C. Cognitive impairment and dementia 20 months after stroke. Neuroepidemiology 23, 45–52 (2004).
Portegies, M. L. P. et al. Prestroke vascular pathology and the risk of recurrent stroke and poststroke dementia. Stroke 47, 2119–2122 (2016).
Ukraintseva, S., Sloan, F., Arbeev, K. & Yashin, A. Increasing rates of dementia at time of declining mortality from stroke. Stroke 37, 1155–1159 (2006).
Farzadfar, F. et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet 377, 578–586 (2011).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 387, 1513–1530 (2016).
NCD Risk Factor Collaboration (NCD-RisC).Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387, 1377–1396 (2016).
Chugh, S. S. et al. Worldwide Epidemiology of Atrial Fibrillation: a Global Burden of Disease 2010 study. Circulation 129, 837–847 (2014).
Kaffashian, S. et al. Long-term clinical impact of vascular brain lesions on magnetic resonance imaging in older adults in the population. Stroke 47, 2865–2869 (2016).
Prins, N. D. et al. Cerebral white matter lesions and the risk of dementia. Arch. Neurol. 61, 1531 (2004).
Mortamais, M. et al. Spatial Distribution of cerebral white matter lesions predicts progression to mild cognitive impairment and dementia. PLoS ONE 8, e56972 (2013).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 389, 37–55 (2017).
Skoog, I. Dementia: Dementia incidence — the times, they are a-changing. Nat. Rev. Neurol. 12, 316–318 (2016).
Choi, J. C. Genetics of cerebral small vessel disease. J. Stroke 17, 7–16 (2015).
Ikram, M. A. et al. Genetics of vascular dementia — review from the ICVD working group. BMC Med. 15, 48 (2017).
Tan, R., Traylor, M., Rutten-Jacobs, L. & Markus, H. New insights into mechanisms of small vessel disease stroke from genetics. Clin. Sci. 131, 515–531 (2017).
Schneider, J. A., Wilson, R. S., Bienias, J. L., Evans, D. A. & Bennett, D. A. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62, 1148–1155 (2004).
Gold, G., Giannakopoulos, P., Herrmann, F. R., Bouras, C. & Kövari, E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 130, 2830–2836 (2007).
Skrobot, O. A. et al. Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain 139, 2957–2969 (2016).
Deramecourt, V. et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology 78, 1043–1050 (2012).
Arvanitakis, Z. et al. The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol. 27, 77–85 (2017).
Lin, W.-L., Castanedes-Casey, M. & Dickson, D. W. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J. Neuropathol. Exp. Neurol. 68, 1167–1176 (2009).
Dudvarski Stankovic, N., Teodorczyk, M., Ploen, R., Zipp, F. & Schmidt, M. H. H. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 131, 347–363 (2016).
Longstreth, W. T. Brain abnormalities in the elderly: frequency and predictors in the United States (the Cardiovascular Health Study). Cardiovascular Health Study Collaborative Research Group. J. Neural Transm. Suppl. 53, 9–16 (1998).
Schneider, J. A. et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 60, 1082–1088 (2003).
Troncoso, J. C. et al. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann. Neurol. 64, 168–176 (2008).
Arvanitakis, Z., Leurgans, S. E., Barnes, L. L., Bennett, D. A. & Schneider, J. A. Microinfarct pathology, dementia, and cognitive systems. Stroke 42, 722–727 (2011).
James, B. D., Bennett, D. A., Boyle, P. A., Leurgans, S. & Schneider, J. A. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA 307, 1798–1800 (2012).
Arvanitakis, Z., Capuano, A. W., Leurgans, S. E., Bennett, D. A. & Schneider, J. A. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 15, 934–943 (2016). This is a detailed study of small and large vessel disease and their role as a mixed pathology with Alzheimer disease pathology that lowers the threshold for dementia even in the absence of infarcts.
Makin, S. D. J., Turpin, S., Dennis, M. S. & Wardlaw, J. M. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J. Neurol. Neurosurg. Psychiatry 84, 893–900 (2013).
Snowdon, D. A. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277, 813–817 (1997).
White, L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J. Alzheimers. Dis. 18, 713–725 (2009).
Sonnen, J. A. et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann. Neurol. 62, 406–413 (2007).
Brundel, M., de Bresser, J., van Dillen, J. J., Kappelle, L. J. & Biessels, G. J. Cerebral microinfarcts: a systematic review of neuropathological studies. J. Cereb. Blood Flow Metab. 32, 425–436 (2012).
Westover, M. B., Bianchi, M. T., Yang, C., Schneider, J. A. & Greenberg, S. M. Estimating cerebral microinfarct burden from autopsy samples. Neurology 80, 1365–1369 (2013).
Smith, E. E. et al. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 11, 272–282 (2012). This is a comprehensive review of the importance of cerebral microinfarcts, which are related to VCI but are poorly recognized in clinical practice.
Okamoto, Y. et al. Cortical microinfarcts in Alzheimer's disease and subcortical vascular dementia. Neuroreport 20, 990–996 (2009).
van Veluw, S. J. et al. Microbleed and microinfarct detection in amyloid angiopathy: a high-resolution MRI-histopathology study. Brain 139, 3151–3162 (2016).
van Veluw, S. J. et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J. Cereb. Blood Flow Metab. 33, 322–329 (2013).
van Veluw, S. J. et al. Cortical microinfarcts on 3T MRI: clinical correlates in memory-clinic patients. Alzheimers Dement. 11, 1500–1509 (2015).
Hilal, S. et al. Cortical cerebral microinfarcts on 3T MRI. Neurology 87, 1583–1590 (2016).
Doyle, K. P. et al. B-Lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci. 35, 2133–2145 (2015).
Jin, W.-N. et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J. Cereb. Blood Flow Metab. 37, 2224–2236 (2017).
Rosenberg, G. A., Bjerke, M. & Wallin, A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke 45, 1531–1538 (2014).
Carare, R. O., Hawkes, C. A., Jeffrey, M., Kalaria, R. N. & Weller, R. O. Review: Cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol. 39, 593–611 (2013).
Wang, M. et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J. Neurosci. 37, 2870–2877 (2017).
Schrag, M. & Greer, D. M. Clinical associations of cerebral microbleeds on magnetic resonance neuroimaging. J. Stroke Cerebrovasc. Dis. 23, 2489–2497 (2014).
Benedictus, M. R. et al. Microbleeds, mortality, and stroke in Alzheimer disease. JAMA Neurol. 72, 539 (2015). This paper shows that cortical microbleeds are associated with an increased risk of stroke-related mortality.
Chung, C.-P. et al. Strictly lobar cerebral microbleeds are associated with cognitive impairment. Stroke 47, 2497–2502 (2016).
Hase, Y., Horsburgh, K., Ihara, M. & Kalaria, R. N. White matter degeneration in vascular and other ageing-related dementias. J. Neurochem. https://doi.org/10.1111/jnc.14271 (2017).
Wharton, S. B., Simpson, J. E., Brayne, C. & Ince, P. G. Age-associated white matter lesions: the MRC Cognitive Function and Ageing Study. Brain Pathol. 25, 35–43 (2015).
Joutel, A. & Chabriat, H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin. Sci. 131, 635–651 (2017).
Boyle, P. A. et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 85, 1930–1936 (2015).
Yu, L. et al. APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol. Aging 36, 2946–2953 (2015).
Hilal, S. et al. Subcortical atrophy in cognitive impairment and dementia. J. Alzheimer' Dis. 48, 813–823 (2015).
Jagust, W. J. et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann. Neurol. 63, 72–80 (2008).
Erten-Lyons, D. et al. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology 81, 977–983 (2013).
Hinman, J. D., Lee, M. D., Tung, S., Vinters, H. V. & Carmichael, S. T. Molecular disorganization of axons adjacent to human lacunar infarcts. Brain 138, 736–745 (2015).
Saggu, R. et al. Astroglial NF-kB contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathol. Commun. 4, 76 (2016).
Udaka, F., Sawada, H. & Kameyama, M. White matter lesions and dementia: MRI-pathological correlation. Ann. NY Acad. Sci. 977, 411–415 (2002).
Chen, A. et al. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-stroke dementia. Brain 139, 242–258 (2016).
Burton, E. et al. Hyperintensities and fronto-subcortical atrophy on MRI are substrates of mild cognitive deficits after stroke. Dement. Geriatr. Cogn. Disord. 16, 113–118 (2003).
Burrows, F. et al. Systemic inflammation affects reperfusion following transient cerebral ischaemia. Exp. Neurol. 277, 252–260 (2016).
Danton, G. H. & Dietrich, W. D. Inflammatory mechanisms after ischemia and stroke. J. Neuropathol. Exp. Neurol. 62, 127–136 (2003).
Adam, N., Kandelman, S., Mantz, J., Chrétien, F. & Sharshar, T. Sepsis-induced brain dysfunction. Expert Rev. Anti. Infect. Ther. 11, 211–221 (2013).
Sudduth, T. L., Powell, D. K., Smith, C. D., Greenstein, A. & Wilcock, D. M. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 33, 708–715 (2013).
Olichney, J. M. et al. Association between severe cerebral amyloid angiopathy and cerebrovascular lesions in Alzheimer disease is not a spurious one attributable to apolipoprotein E4. Arch. Neurol. 57, 869–874 (2000).
Bell, R. D. & Zlokovic, B. V. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 118, 103–113 (2009).
Faraco, G. et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Invest. 126, 4674–4689 (2016).
Montagne, A. et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Bakker, E. N. T. P. et al. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell. Mol. Neurobiol. 36, 181–194 (2016).
Tarantini, S., Tran, C. H. T., Gordon, G. R., Ungvari, Z. & Csiszar, A. Impaired neurovascular coupling in aging and Alzheimer's disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 94, 52–58 (2016).
Farkas, E. et al. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 108, 57–64 (2004).
Michaud, M. et al. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 14, 877–882 (2013).
Ciolli, L. et al. The VAS-COG clinic: an out-patient service for patients with cognitive and behavioral consequences of cerebrovascular diseases. Neurol. Sci. 33, 1277–1283 (2012).
McKhann, G. et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–944 (1984).
Chui, H. C. et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 42, 473 (1992).
World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Clinical descriptions and diagnostic guidelines (WHO, Geneva, 1992)
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th edn (American Psychiatric Association, 1994).
Pohjasvaara, T., Mantyla, R., Ylikoski, R., Kaste, M. & Erkinjuntti, T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. Stroke 31, 2952–2957 (2000).
Pantoni, L., Garcia, J. H. & Brown, G. G. Vascular pathology in three cases of progressive cognitive deterioration. J. Neurol. Sci. 135, 131–139 (1996).
Staekenborg, S. S. et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke 39, 317–322 (2007).
Erkinjuntti, T. et al. in Advances in Dementia Research (eds Jellinger, K., Schmidt, R. & Windisch, M. ) 23–30 (Springer, 2000).
Bastos-Leite, A. J. et al. The contribution of medial temporal lobe atrophy and vascular pathology to cognitive impairment in vascular dementia. Stroke 38, 3182–3185 (2007).
Staekenborg, S. S. et al. Behavioural and psychological symptoms in vascular dementia; differences between small- and large-vessel disease. J. Neurol. Neurosurg. Psychiatry 81, 547–551 (2009).
Hachinski, V. et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37, 2220–2241 (2006).
Skrobot, O. A. et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. https://doi.org/10.1016/j.jalz.2017.09.007 (2017). This study describes protocols for the diagnosis of VCI based on the results of a Delphi consensus study, conducted in a large, multinational group of researchers, that aimed to achieve consensus on clinical diagnosis of VCI.
METACOHORTS Consortium. METACOHORTS for the study of vascular disease and its contribution to cognitive decline and neurodegeneration: an initiative of the Joint Programme for Neurodegenerative Disease Research. Alzheimers Dement. 12, 1235–1249 (2016).
Sachdev, P. S. et al. STROKOG (Stroke and Cognition Consortium): an international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimers Dement. 7, 11–23 (2017).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Salvadori, E. et al. Development and psychometric properties of a neuropsychological battery for mild cognitive impairment with small vessel disease: the VMCI-Tuscany Study. J. Alzheimers. Dis. 43, 1313–1323 (2015).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Sorbi, S. et al. EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur. J. Neurol. 19, 1159–1179 (2012).
Schoonenboom, N. S. M. et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 78, 47–54 (2012).
Wallin, A. et al. Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease — a consensus report. BMC Neurol. 17, 102 (2017).
Kalaria, R. N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer's disease. Acta Neuropathol. 131, 659–685 (2016).
Pantoni, L. et al. Postmortem examination of vascular lesions in cognitive impairment: a survey among neuropathological services. Stroke 37, 1005–1009 (2006).
Kalaria, R. N. & Ihara, M. Medial temporal lobe atrophy is the norm in cerebrovascular dementias. Eur. J. Neurol. 24, 539–540 (2017).
de Bruijn, R. F. A. G. et al. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med. 13, 132 (2015).
Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K. & Brayne, C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 13, 788–794 (2014).
Moll van Charante, E. P. et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 388, 797–805 (2016).
Dichgans, M. & Zietemann, V. Prevention of vascular cognitive impairment. Stroke 43, 3137–3146 (2012).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015).
Smith, E. E. et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48, e44–e71 (2017). This paper summarizes the evidence on the diagnosis and management of silent cerebrovascular disease to prevent stroke and concludes that primary stroke prevention is indicated in patients with silent brain infarcts, WMHs or microbleeds.
Kernan, W. N. et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 45, 2160–2236 (2014).
Mok, V. C. T. et al. Early-onset and delayed-onset poststroke dementia — revisiting the mechanisms. Nat. Rev. Neurol. 13, 148–159 (2017).
Pendlebury, S. T. & Rothwell, P. M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 8, 1006–1018 (2009).
Tzourio, C. et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 163, 1069–1075 (2003).
Diener, H.-C. et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 7, 875–884 (2008).
Pearce, L. A. et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol. 13, 1177–1185 (2014).
Mok, V. C. T. et al. Delayed-onset dementia after stroke or transient ischemic attack. Alzheimers Dement. 12, 1167–1176 (2016).
Douiri, A., McKevitt, C., Emmett, E. S., Rudd, A. G. & Wolfe, C. D. A. Long-term effects of secondary prevention on cognitive function in stroke patients. Circulation 128, 1341–1348 (2013).
The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 373, 2103–2116 (2015).
[No authors listed.] Systolic Blood Pressure Intervention Trial (SPRINT) Overview. National Heart, Lung and Blood Institutehttps://www.nhlbi.nih.gov/news/systolic-blood-pressure-intervention-trial-sprint-overview (2017).
Birns, J. & Kalra, L. Cognitive function and hypertension. J. Hum. Hypertens. 23, 86–96 (2008).
Thoonsen, H. et al. Aspirin in Alzheimer's disease: increased risk of intracerebral hemorrhage: cause for concern? Stroke 41, 2690–2692 (2010).
Van der Flier, W. M. & Cordonnier, C. Microbleeds in vascular dementia: clinical aspects. Exp. Gerontol. 47, 853–857 (2012).
Cordonnier, C. & van der Flier, W. M. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain 134, 335–344 (2011).
Wang, Z., Soo, Y. O. Y. & Mok, V. C. T. Cerebral microbleeds. Stroke 45, 2811–2817 (2014).
Jacobs, V. et al. Long-term population-based cerebral ischemic event and cognitive outcomes of direct oral anticoagulants compared with warfarin among long-term anticoagulated patients for atrial fibrillation. Am. J. Cardiol. 118, 210–214 (2016).
Matz, K. et al. Multidomain lifestyle interventions for the prevention of cognitive decline after ischemic stroke. Stroke 46, 2874–2880 (2015).
Benjamin, P. et al. Progression of MRI markers in cerebral small vessel disease: sample size considerations for clinical trials. J. Cereb. Blood Flow Metab. 36, 228–240 (2016).
Schmidt, R. et al. White matter lesion progression in LADIS: frequency, clinical effects, and sample size calculations. Stroke 43, 2643–2647 (2012).
Prins, N. D. & Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 11, 157–165 (2015).
Inzitari, D. et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 339, b2477 (2009). This study shows the independent contribution of WMHs detected by MRI to loss of independence and risk of mortality.
Gouw, A. A. et al. On the etiology of incident brain lacunes: longitudinal observations from the lADIS study. Stroke 39, 3083–3085 (2008).
Gouw, A. A. et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke 39, 1414–1420 (2008).
Dufouil, C. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging substudy. Circulation 112, 1644–1650 (2005).
Hasegawa, Y. et al. Effects of perindopril-based blood pressure lowering and of patient characteristics on the progression of silent brain infarct: the Perindopril Protection against Recurrent Stroke Study (PROGRESS) CT substudy in Japan. Hypertens. Res. 27, 147–156 (2004).
van Dijk, E. J. et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 39, 2712–2719 (2008).
Mok, V. C. T. et al. Effects of statins on the progression of cerebral white matter lesion. J. Neurol. 256, 750–757 (2009).
Fu, J. H. et al. Effects of statins on progression of subclinical brain infarct. Cerebrovasc. Dis. 30, 51–56 (2010).
Xiong, Y. et al. Prestroke statins, progression of white matter hyperintensities, and cognitive decline in stroke patients with confluent white matter hyperintensities. Neurotherapeutics 11, 606–611 (2014).
ten Dam, V. H. et al. Effect of pravastatin on cerebral infarcts and white matter lesions. Neurology 64, 1807–1809 (2005).
Cavalieri, M. et al. B vitamins and magnetic resonance imaging-detected ischemic brain lesions in patients with recent transient ischemic attack or stroke: The VITAmins TO Prevent Stroke (VITATOPS) MRI-substudy. Stroke 43, 3266–3270 (2012).
VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 9, 855–865 (2010).
Gopalan, Y. et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke 45, 1422–1428 (2014).
Richard, E., Gouw, A. A., Scheltens, P. & van Gool, W. A. Vascular care in patients with alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the Evaluation of Vascular Care in Alzheimer's Disease (EVA) Study. Stroke 41, 554–556 (2010).
Leeuwis, A. E. et al. Design of the ExCersion-VCI study: the effect of aerobic exercise on cerebral perfusion in patients with vascular cognitive impairment. Alzheimers Dement. 3, 157–165 (2017).
Cyarto, E. V. et al. Protocol for a randomized controlled trial evaluating the effect of physical activity on delaying the progression of white matter changes on MRI in older adults with memory complaints and mild cognitive impairment: the AIBL Active trial. BMC Psychiatry 12, 167 (2012).
Baykara, E. et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann. Neurol. 80, 581–592 (2016).
Yang, J. et al. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimers Dement. 11, 16–23 (2015).
Lee, J. H. et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology 77, 18–25 (2011).
Liu, W. et al. Influence of amyloid-β on cognitive decline after stroke/transient ischemic attack. Stroke 46, 3074–3080 (2015).
Scheltens, P. et al. Alzheimer's disease. Lancet 388, 505–517 (2016).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 537, 50–56 (2016).
Kavirajan, H. & Schneider, L. S. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol. 6, 782–792 (2007).
Roman, G. C. et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke 41, 1213–1221 (2010).
Mbius, H. J. & Stffler, A. Memantine in vascular dementia. Int. Psychogeriatr. 15, 207–213 (2003).
Guekht, A., Skoog, I., Edmundson, S., Zakharov, V. & Korczyn, A. D. ARTEMIDA Trial (a randomized trial of efficacy, 12 months international double-blind actovegin). Stroke 48, 1262–1270 (2017).
Chen, N. et al. Cerebrolysin for vascular dementia. Cochrane Database Syst. Rev. 1, CD008900 (2013)
Pantoni, L. et al. Efficacy and safety of nimodipine in subcortical vascular dementia: a randomized placebo-controlled trial. Stroke 36, 619–624 (2005).
Jia, J. et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement. 12, 89–99 (2016).
Napryeyenko, O., Sonnik, G. & Tartakovsky, I. Efficacy and tolerability of Ginkgo biloba extract EGb 761 by type of dementia: analyses of a randomised controlled trial. J. Neurol. Sci. 283, 224–229 (2009).
Ihl, R., Tribanek, M., Bachinskaya, N. & GOTADAY Study Group. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761® in Alzheimer's disease and vascular dementia: results from a randomised controlled trial. Pharmacopsychiatry 45, 41–46 (2012).
Yuan, Q., Wang, C., Shi, J. & Lin, Z. Effects of Ginkgo biloba on dementia: an overview of systematic reviews. J. Ethnopharmacol. 195, 1–9 (2017).
Tang, Y. et al. The efficacy of Cognitive training in patients with VAsCular Cognitive Impairment, No dEmentia (the Cog-VACCINE study): study protocol for a randomized controlled trial. Trials 17, 392 (2016).
Guerra, A. et al. Transcranial magnetic stimulation studies in Alzheimer's disease. Int. J. Alzheimers. Dis. 2011, 1–9 (2011).
Kubis, N. Non-invasive brain stimulation to enhance post-stroke recovery. Front. Neural Circuits 10, 56 (2016).
Baker, E. W. et al. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci. Rep. 7, 10075 (2017).
Bang, O. Y., Kim, E. H., Cha, J. M. & Moon, G. J. Adult stem cell therapy for stroke: challenges and progress. J. Stroke 18, 256–266 (2016).
de Hert, M., Schreurs, V., Vancampfort, D. & van Winkel, R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry 8, 15–22 (2009).
Wang, P. S. et al. Risk of death in elderly users of conventional versus atypical antipsychotic medications. N. Engl. J. Med. 353, 2335–2341 (2005).
Anderson, I. M. & Tomenson, B. M. Treatment discontinuation with selective serotonin reuptake inhibitors compared with tricyclic antidepressants: a meta-analysis. BMJ 310, 1433–1438 (1995).
Bondon-Guitton, E. et al. Drug-induced parkinsonism: a review of 17 years’ experience in a regional pharmacovigilance center in France. Mov. Disord. 26, 2226–2231 (2011).
Korczyn, A. D. Vascular parkinsonism — characteristics, pathogenesis and treatment. Nat. Rev. Neurol. 11, 319–326 (2015).
[No authors listed.] The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc. Sci. Med. 41, 1403–1409 (1995).
Bowling, A. et al. Quality of life in dementia: a systematically conducted narrative review of dementia-specific measurement scales. Aging Ment. Health 19, 13–31 (2014). This is a recent and comprehensive review of QOL scales for dementia.
Logsdon, R. G., Gibbons, L. E., McCurry, S. M. & Teri, L. Assessing quality of life in older adults with cognitive impairment. Psychosom. Med. 64, 510–519 (2002).
Brod, M., Stewart, A. L., Sands, L. & Walton, P. Conceptualization and measurement of quality of life in dementia: the Dementia Quality of Life Instrument (DQoL). Gerontologist 39, 25–36 (1999).
Weiner, M. F. et al. The quality of life in late-stage dementia (QUALID) scale. J. Am. Med. Dir. Assoc. 1, 114–116 (2000).
Ettema, T. P., Dröes, R.-M., de Lange, J., Mellenbergh, G. J. & Ribbe, M. W. QUALIDEM: development and evaluation of a dementia specific quality of life instrument — validation. Int. J. Geriatr. Psychiatry 22, 424–430 (2007).
Williams, L. S., Weinberger, M., Harris, L. E., Clark, D. O. & Biller, J. Development of a stroke-specific quality of life scale. Stroke 30, 1362–1369 (1999).
Lawton, M. P. Quality of life in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 8, 138–150 (1994).
Etters, L., Goodall, D. & Harrison, B. E. Caregiver burden among dementia patient caregivers: a review of the literature. J. Am. Acad. Nurse Pract. 20, 423–428 (2008).
Thomas, P. et al. Dementia patients caregivers quality of life: the PIXEL study. Int. J. Geriatr. Psychiatry 21, 50–56 (2006).
Belle, S. H. et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann. Intern. Med. 145, 727–738 (2006).
Graff, M. J. L. et al. Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: a randomized controlled trial. Journals Gerontol. Ser. A 62, 1002–1009 (2007).
van der Flier, W. M. et al. Interaction of medial temporal lobe atrophy and white matter hyperintensities in AD. Neurology 62, 1862–1864 (2004).
Yarchoan, M. et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 135, 3749–3756 (2012).
Beach, T. G. et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 113, 13–21 (2006).
Barnes, J. et al. Vascular and Alzheimer's disease markers independently predict brain atrophy rate in Alzheimer's Disease Neuroimaging Initiative controls. Neurobiol. Aging 34, 1996–2002 (2013).
Goos, J. D. C. et al. Patients with Alzheimer disease with multiple microbleeds. Stroke 40, 3455–3460 (2009).
Author information
Authors and Affiliations
Contributions
Introduction (W.M.v.d.F.); Epidemiology (I.S.); Mechanisms/pathophysiology (J.A.S.); Diagnosis, screening and prevention (L.P. and P.S.); Management (V.M.); Quality of life (C.L.H.C.); Outlook (All); Overview of Primer (W.M.v.d.F. and P.S.).
Corresponding author
Ethics declarations
Competing interests
W.M.v.d.F. has been an invited speaker at Boehringer Ingelheim and has received grant support from Boehringer Ingelheim, Biogen MA Inc, Piramal Neuroimaging, Roche BV, Janssen Stellar and Combinostics. All funding is paid to her institution. I.S. has been a consultant for Takeda and has given paid lectures for Takeda in relation to vascular dementia. J.A.S. has been on the scientific advisory boards of Genentech, Eli Lilly and Grifols and has received consultancy fees from Navidea Biopharmaceuticals and the Michael J. Fox Foundation. C.L.H.C. has received research support from Moleac, Nutricia, Lundbeck, Eisai, GlaxoSmithKline and Merck. All funding is paid to his institution. P.S. has acquired grant support from GE Healthcare, Danone Research, Piramal and Merck and, in the past 2 years, has received consultancy and speaker fees from GE Healthcare, Novartis, Nutricia, Probiodrug, Biogen, Lundbeck, Roche and EIP Pharma. All funding is paid to his institution. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
van der Flier, W., Skoog, I., Schneider, J. et al. Vascular cognitive impairment. Nat Rev Dis Primers 4, 18003 (2018). https://doi.org/10.1038/nrdp.2018.3
Published:
DOI: https://doi.org/10.1038/nrdp.2018.3
This article is cited by
-
The associations between peripheral inflammatory and lipid parameters, white matter hyperintensity, and cognitive function in patients with non-disabling ischemic cerebrovascular events
BMC Neurology (2024)
-
Selective vulnerability of hippocampal sub-regions in patients with subcortical vascular mild cognitive impairment
Brain Imaging and Behavior (2024)
-
Vaskuläre Demenz
DGNeurologie (2024)
-
Correlation Between Cognitive Impairment and Lenticulostriate Arteries: A Clinical and Radiomics Analysis
Journal of Imaging Informatics in Medicine (2024)
-
Binary Nano-inhalant Formulation of Icariin Enhances Cognitive Function in Vascular Dementia via BDNF/TrkB Signaling and Anti-inflammatory Effects
Neurochemical Research (2024)