Key Points
-
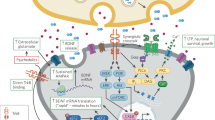
Brain-derived neurotrophic factor (BDNF) is widely produced in the cortex throughout life, where it influences neuronal function. Levels of BDNF become deficient in the cerebral cortex in Alzheimer's disease.
-
In animal models of Alzheimer's disease, BDNF exhibits potent therapeutic effects that include prevention of cell death, stimulation of neuronal function, improvement in synaptic markers and improvements in learning and memory. Accordingly, BDNF represents a potentially promising therapeutic avenue in Alzheimer's disease.
-
Other neurological and psychiatric disorders — for example, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and depression — could also respond to BDNF treatment.
-
Therapeutic BDNF delivery to the brain is a major challenge. BDNF does not readily cross the blood–brain barrier, and widespread central administration causes intolerable adverse effects. Localized and sustained delivery of the growth factor will be required to treat many neurological disorders.
-
Gene therapy may be a useful method of delivering BDNF to specific brain regions in neurological disorders. For example, clinical trials involving BDNF gene delivery to the entorhinal and/or the hippocampal circuitry regions in Alzheimer's disease are planned.
-
Other methods for increasing BDNF levels in the brain include the use of small peptide mimetics, drug-induced increases in BDNF and even exercise. It remains to be established, however, whether these methods can induce sufficient increases in BDNF levels to effectively treat neurological diseases.
Abstract
The growth factor brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin-related kinase receptor type B (TRKB) are actively produced and trafficked in multiple regions in the adult brain, where they influence neuronal activity, function and survival throughout life. The diverse presence and activity of BDNF suggests a potential role for this molecule in the pathogenesis and treatment of both neurological and psychiatric disorders. This article reviews the current understanding and future directions in BDNF-related research in the central nervous system, with an emphasis on the possible therapeutic application of BDNF in modifying fundamental processes underlying neural disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Levi-Montalcini, R. & Hamburger, V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 116, 321–361 (1951).
Johnson, E. M. Jr & Tuszynski, M. H. in CNS Regeneration (eds. Kordower, J. H. & Tuszynski, M. H.) 95–144 (Academic Press, San Diego, 2008).
Barde, Y. A., Edgar, D. & Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1, 549–553 (1982). This landmark article presented the discovery of BDNF.
Mowla, S. J. et al. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 276, 12660–12666 (2001).
Mowla, S. J. et al. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J. Neurosci. 19, 2069–2080 (1999).
Lee, R., Kermani, P., Teng, K. K. & Hempstead, B. L. Regulation of cell survival by secreted proneurotrophins. Science 294, 1945–1948 (2001).
Nikoletopoulou, V. et al. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature 467, 59–63 (2010).
Rauskolb, S. et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J. Neurosci. 30, 1739–1749 (2010).
Matsumoto, T. et al. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nature Neurosci. 11, 131–133 (2008).
Soppet, D. et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 65, 895–903 (1991).
Carter, B. D. et al. Selective activation of NF-κB by nerve growth factor through the neurotrophin receptor p75. Science 272, 542–545 (1996).
Tanaka, J. et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319, 1683–1687 (2008).
Horch, H. W. & Katz, L. C. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nature Neurosci. 5, 1177–1184 (2002).
Figurov, A., Pozzo-Miller, L. D., Olafsson, P., Wang, T. & Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381, 706–709 (1996).
Kang, H. & Schuman, E. M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662 (1995).
Yamada, K., Mizuno, M. & Nabeshima, T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 70, 735–744 (2002).
Egan, M. F. et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 (2003). An important study that described a Val66Met BDNF gene polymorphism that is associated with memory deficits and altered hippocampal function.
Cathomas, F., Vogler, C., Euler-Sigmund, J. C., de Quervain, D. J. & Papassotiropoulos, A. Fine-mapping of the brain-derived neurotrophic factor (BDNF) gene supports an association of the Val66Met polymorphism with episodic memory. Int. J. Neuropsychopharmacol. 13, 975–980 (2010).
Xu, B. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature Neurosci. 6, 736–742 (2003).
Cao, L. et al. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nature Med. 15, 447–454 (2009). This study used gene delivery techniques to control BDNF expression in an animal model of obesity.
Nagahara, A. H. et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature Med. 15, 331–337 (2009). This article demonstrated the therapeutic effects of BDNF in six animal models of Alzheimer's disease, ranging from mouse models to non-human primate models.
Smith, D. E., Roberts, J., Gage, F. H. & Tuszynski, M. H. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc. Natl Acad. Sci. USA 96, 10893–10898 (1999).
Kordower, J. H. et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290, 767–773 (2000).
Tuszynski, M. H. et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature Med. 11, 551–555 (2005). A report on the first clinical trial of growth factor gene therapy in a human neurodegenerative disorder: Alzheimer's disease.
Bradley, W. G. A phase I/II study of recombinant brain-derived neurotrophic factor in patients with ALS. Ann. Neurol. 38, 971 (1995).
Apfel, S. C. et al. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. NGF Study Group. Neurology 51, 695–702 (1998).
Kordower, J. H. et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Ann. Neurol. 46, 419–424 (1999).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 82, 239–259 (1991).
Kordower, J. H. et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 49, 202–213 (2001).
Masliah, E. et al. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci. Lett. 174, 67–72 (1994).
Yan, Q. et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 78, 431–448 (1997).
Altar, C. A. et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389, 856–860 (1997).
Kovalchuk, Y., Hanse, E., Kafitz, K. W. & Konnerth, A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science 295, 1729–1734 (2002).
Connor, B. et al. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res. Mol. Brain Res. 49, 71–81 (1997).
Hock, C., Heese, K., Hulette, C., Rosenberg, C. & Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 57, 846–851 (2000).
Narisawa-Saito, M., Wakabayashi, K., Tsuji, S., Takahashi, H. & Nawa, H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer's disease. Neuroreport 7, 2925–2928 (1996).
Mucke, L. et al. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Burbach, G. J. et al. Induction of brain-derived neurotrophic factor in plaque-associated glial cells of aged APP23 transgenic mice. J. Neurosci. 24, 2421–2430 (2004).
Blurton-Jones, M. et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl Acad. Sci. USA 106, 13594–13599 (2009). An important paper indicating that BDNF that is secreted by transplanted embryonic stem cells exerts therapeutic effects in a mouse model of Alzheimer's disease.
Olanow, C. W. & Tatton, W. G. Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 22, 123–144 (1999).
Hyman, C. et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350, 230–232 (1991).
Mogi, M. et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson's disease. Neurosci. Lett. 270, 45–48 (1999).
Parain, K. et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson's disease substantia nigra. Neuroreport 10, 557–561 (1999).
Levivier, M., Przedborski, S., Bencsics, C. & Kang, U. J. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson's disease. J. Neurosci. 15, 7810–7820 (1995).
Frim, D. M. et al. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc. Natl Acad. Sci. USA 91, 5104–5108 (1994).
Tsukahara, T., Takeda, M., Shimohama, S., Ohara, O. & Hashimoto, N. Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- induced parkinsonism in monkeys. Neurosurgery 37, 733–741 (1995).
Orr, H. T. & Zoghbi, H. Y. Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30, 575–621 (2007).
Walker, F. O. Huntington's disease. Lancet 369, 218–228 (2007).
Zuccato, C. et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science 293, 493–498 (2001).
Baquet, Z. C., Gorski, J. A. & Jones, K. R. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J. Neurosci. 24, 4250–4258 (2004).
Gauthier, L. R. et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118, 127–138 (2004).
Strand, A. D. et al. Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J. Neurosci. 27, 11758–11768 (2007).
Zala, D. et al. Progressive and selective striatal degeneration in primary neuronal cultures using lentiviral vector coding for a mutant huntingtin fragment. Neurobiol. Dis. 20, 785–798 (2005).
Canals, J. M. et al. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J. Neurosci. 24, 7727–7739 (2004).
Simmons, D. A. et al. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc. Natl Acad. Sci. USA 106, 4906–4911 (2009). This article reported that ampakines increase BDNF expression in the brain and reduce deficits in a transgenic mouse model of Huntington's disease.
Kells, A. P., Henry, R. A. & Connor, B. AAV–BDNF mediated attenuation of quinolinic acid-induced neuropathology and motor function impairment. Gene Ther. 15, 966–977 (2008).
Kaplan, D. R. & Miller, F. D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 10, 381–391 (2000).
Wijesekera, L. C. & Leigh, P. N. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 4, 3 (2009).
Tuszynski, M. H., Mafong, E. & Meyer, S. BDNF and NT-4/5 prevent injury-induced motor neuron degeneration in the adult central nervous system. Neuroscience 71, 761–771 (1996).
Giehl, K. M. & Tetzlaff, W. BDNF and NT-3, but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur. J. Neurosci. 8, 1167–1175 (1996).
Mitsumoto, H. et al. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science 265, 1107–1110 (1994).
[No authors listed.] A controlled trial of recombinant methionyl human BDNF in ALS: the BDNF study group (Phase III). Neurology 52, 1427–1433 (1999). This was a major report on a Phase III multicentre trial of BDNF protein administration in a neurodegenerative disease: ALS.
Beck, M. et al. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 6, 100–103 (2005).
Ochs, G. et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 201–206 (2000).
Ankeny, D. P. et al. Pegylated brain-derived neurotrophic factor shows improved distribution into the spinal cord and stimulates locomotor activity and morphological changes after injury. Exp. Neurol. 170, 85–100 (2001).
Pardridge, W. M., Wu, D. & Sakane, T. Combined use of carboxyl-directed protein pegylation and vector-mediated blood–brain barrier drug delivery system optimizes brain uptake of brain-derived neurotrophic factor following intravenous administration. Pharm. Res. 15, 576–582 (1998).
Doyle, K. P., Simon, R. P. & Stenzel-Poore, M. P. Mechanisms of ischemic brain damage. Neuropharmacology 55, 310–318 (2008).
Ferrer, I. et al. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 101, 229–238 (2001).
Muller, H. D. et al. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke 39, 1012–1021 (2008).
Larsson, E., Nanobashvili, A., Kokaia, Z. & Lindvall, O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J. Cereb. Blood Flow Metab. 19, 1220–1228 (1999).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Gordon, T., Sulaiman, O. & Boyd, J. G. Experimental strategies to promote functional recovery after peripheral nerve injuries. J. Peripher. Nerv. Syst. 8, 236–250 (2003).
Tobias, C. A. et al. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp. Neurol. 184, 97–113 (2003).
Blesch, A. & Tuszynski, M. H. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J. Neurosci. 27, 10535–10545 (2007).
Lu, P., Blesch, A. & Tuszynski, M. H. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J. Comp. Neurol. 436, 456–470 (2001).
Stokols, S. & Tuszynski, M. H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 27, 443–451 (2006).
Blesch, A. & Tuszynski, M. H. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 32, 41–47 (2009).
Alto, L. T. et al. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nature Neurosci. 12, 1106–1113 (2009).
Hollis, E. R., Jamshidi, P., Low, K., Blesch, A. & Tuszynski, M. H. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc. Natl Acad. Sci. USA 106, 7215–7220 (2009).
Plunet, W. T. et al. Dietary restriction started after spinal cord injury improves functional recovery. Exp. Neurol. 213, 28–35 (2008).
Ying, Z. et al. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 155, 1070–1078 (2008).
Schumacher, J. et al. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol. Psychiatry 58, 307–314 (2005).
Post, R. M. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. J. Psychiatr. Res. 41, 979–990 (2007).
Kato, M. & Serretti, A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol. Psychiatry 15, 473–500 (2010).
Campbell, S., Marriott, M., Nahmias, C. & MacQueen, G. M. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry 161, 598–607 (2004).
Dwivedi, Y. et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 60, 804–815 (2003).
Brunoni, A. R., Lopes, M. & Fregni, F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 11, 1169–1180 (2008).
Vermetten, E., Vythilingam, M., Southwick, S. M., Charney, D. S. & Bremner, J. D. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry 54, 693–702 (2003).
Sen, S., Duman, R. & Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol. Psychiatry 64, 527–532 (2008).
Shimizu, E. et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 54, 70–75 (2003).
Chen, B., Dowlatshahi, D., MacQueen, G. M., Wang, J. F. & Young, L. T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 50, 260–265 (2001).
Cotman, C. W. & Berchtold, N. C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301 (2002).
Neeper, S. A., Gomez-Pinilla, F., Choi, J. & Cotman, C. W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 726, 49–56 (1996).
Fuss, J. et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus 20, 364–376 (2010).
Shimizu, E., Hashimoto, K. & Iyo, M. Ethnic difference of the BDNF 196G/A (val166met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 126B, 122–123 (2004).
Dempster, E. et al. Association between BDNF val66 met genotype and episodic memory. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B, 73–75 (2005).
Hariri, A. R. et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 23, 6690–6694 (2003).
Pezawas, L. et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 24, 10099–10102 (2004).
Erickson, K. I. et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 30, 5368–5375 (2010).
Rybakowski, J. K. BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics 9, 1589–1593 (2008).
Sugiyama, N., Kanba, S. & Arita, J. Temporal changes in the expression of brain-derived neurotrophic factor mRNA in the ventromedial nucleus of the hypothalamus of the developing rat brain. Brain Res. Mol. Brain Res. 115, 69–77 (2003).
Pelleymounter, M. A., Cullen, M. J. & Wellman, C. L. Characteristics of BDNF-induced weight loss. Exp. Neurol. 131, 229–238 (1995).
Fox, E. A. & Byerly, M. S. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R994–R1004 (2004).
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15, 1748–1757 (2001).
Gray, J. et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55, 3366–3371 (2006).
Yeo, G. S. et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nature Neurosci. 7, 1187–1189 (2004).
Tonra, J. R. et al. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Leprdb/leprdb mice. Diabetes 48, 588–594 (1999).
Ono, M. et al. Intermittent administration of brain-derived neurotrophic factor ameliorates glucose metabolism in obese diabetic mice. Metabolism 49, 129–133 (2000).
Fujinami, A. et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: relationship to glucose metabolism and biomarkers of insulin resistance. Clin. Biochem. 41, 812–817 (2008).
Winkler, J. et al. Reversible Schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Ann. Neurol. 41, 82–93 (1997).
Williams, L. R. Hypophagia is induced by intracerebroventricular administration of nerve growth factor. Exp. Neurol. 113, 31–37 (1991).
Eriksdotter Jonhagen, M. et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 9, 246–257 (1998).
Isaacson, L. G., Saffran, B. N. & Crutcher, K. A. Intracerebral NGF infusion induces hyperinnervation of cerebral blood vessels. Neurobiol. Aging 11, 51–55 (1990).
Nutt, J. G. et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60, 69–73 (2003).
Lang, A. E. et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 59, 459–466 (2006).
Hovland, D. N. Jr et al. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF in rhesus monkeys. Toxicol. Pathol. 35, 1013–1029 (2007).
Gasmi, M. et al. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson's disease. Mol. Ther. 15, 62–68 (2007).
Christine, C. W. et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73, 1662–1669 (2009).
Feigin, A. et al. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson's disease. Proc. Natl Acad. Sci. USA 104, 19559–19564 (2007).
Marks, W. J. Jr et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 7, 400–408 (2008). This article reported the results of gene delivery of the growth factor neurturin in a clinical trial of Parkinson's disease.
Worgall, S. et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 19, 463–474 (2008).
Marks, W. J. Jr et al. Gene delivery of AAV2–neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 9, 1164–1172 (2010).
Herzog, C. D. et al. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson's disease. Neurosurgery 64, 602–613 (2009).
Fiandaca, M. S. et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage 47 (Suppl. 2), 27–35 (2009).
Martinowich, K. et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302, 890–893 (2003).
Roth, T. L., Lubin, F. D., Funk, A. J. & Sweatt, J. D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 65, 760–769 (2009).
Lubin, F. D., Roth, T. L. & Sweatt, J. D. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J. Neurosci. 28, 10576–10586 (2008).
Ma, D. K. et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077 (2009).
Tsankova, N. M. et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neurosci. 9, 519–525 (2006).
Young, W. Review of lithium effects on brain and blood. 18, 951–975 Cell Transplant. (2009).
Drzyzga, L. R., Marcinowska, A. & Obuchowicz, E. Antiapoptotic and neurotrophic effects of antidepressants: a review of clinical and experimental studies. Brain Res. Bull. 79, 248–257 (2009).
Quiroz, J. A., Machado-Vieira, R., Zarate, C. A. Jr & Manji, H. K. Novel insights into lithium's mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology 62, 50–60 (2010).
Leyhe, T. et al. Increase of BDNF serum concentration in lithium treated patients with early Alzheimer's disease. J. Alzheimers Dis. 16, 649–656 (2009).
Adessi, C. & Soto, C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr. Med. Chem. 9, 963–978 (2002).
O'Leary, P. D. & Hughes, R. A. Design of potent peptide mimetics of brain-derived neurotrophic factor. J. Biol. Chem. 278, 25738–25744 (2003).
Longo, F. M. et al. Small molecule neurotrophin receptor ligands: novel strategies for targeting Alzheimer's disease mechanisms. Curr. Alzheimer Res. 4, 503–506 (2007).
Price, R. D., Milne, S. A., Sharkey, J. & Matsuoka, N. Advances in small molecules promoting neurotrophic function. Pharmacol. Ther. 115, 292–306 (2007).
Vaynman, S., Ying, Z. & Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 20, 2580–2590 (2004).
Lee, J., Duan, W., Long, J. M., Ingram, D. K. & Mattson, M. P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 15, 99–108 (2000).
Molteni, R., Barnard, R. J., Ying, Z., Roberts, C. K. & Gomez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 112, 803–814 (2002).
Maswood, N. et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc. Natl Acad. Sci. USA 101, 18171–18176 (2004).
Duan, W. & Mattson, M. P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J. Neurosci. Res. 57, 195–206 (1999).
Duan, W., Guo, Z., Jiang, H., Ware, M. & Mattson, M. P. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 144, 2446–2453 (2003).
Pang, T. Y., Stam, N. C., Nithianantharajah, J., Howard, M. L. & Hannan, A. J. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience 141, 569–584 (2006).
Nichol, K., Deeny, S. P., Seif, J., Camaclang, K. & Cotman, C. W. Exercise improves cognition and hippocampal plasticity in APOE ɛ4 mice. Alzheimers Dement. 5, 287–294 (2009).
Goverdhana, S. et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol. Ther. 12, 189–211 (2005).
Su, X. et al. Real-time MR imaging with gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol. Ther. 18, 1490–1495 (2010).
Massa, S. M. et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Invest. 120, 1774–1785 (2010). A recent study that revealed the potential effects of small peptide mimetics of BDNF in neurodegenerative disease models.
Yan, Q. et al. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 378, 135–157 (1997).
Lu, B., Buck, C. R., Dreyfus, C. F. & Black, I. B. Expression of NGF and NGF receptor mRNAs in the developing brain: evidence for local delivery and action of NGF. Exp. Neurol. 104, 191–199 (1989).
Koshimizu, H., Hazama, S., Hara, T., Ogura, A. & Kojima, M. Distinct signaling pathways of precursor BDNF and mature BDNF in cultured cerebellar granule neurons. Neurosci. Lett. 473, 229–232 (2010).
Armanini, M. P., McMahon, S. B., Sutherland, J., Shelton, D. L. & Phillips, H. S. Truncated and catalytic isoforms of trkB are co-expressed in neurons of rat and mouse CNS. Eur. J. Neurosci. 7, 1403–1409 (1995).
Ohira, K. et al. Truncated TrkB-T1 regulates the morphology of neocortical layer I astrocytes in adult rat brain slices. Eur. J. Neurosci. 25, 406–416 (2007).
Huang, E. J. & Reichardt, L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003).
Zheng, J. et al. Clathrin-dependent endocytosis is required for TrkB-dependent Akt-mediated neuronal protection and dendritic growth. J. Biol. Chem. 283, 13280–13288 (2008).
Howe, C. L., Valletta, J. S., Rusnak, A. S. & Mobley, W. C. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras–MAPK pathway. Neuron 32, 801–814 (2001).
Sharma, N. et al. Long-distance control of synapse assembly by target-derived NGF. Neuron 67, 422–434 (2010).
Kafitz, K. W., Rose, C. R., Thoenen, H. & Konnerth, A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 401, 918–921 (1999).
Blum, R., Kafitz, K. W. & Konnerth, A. Neurotrophin-evoked depolarization requires the sodium channel NaV1.9. Nature 419, 687–693 (2002).
Tuszynski, M. H. Growth factor gene delivery: animal models to clinical trials. Dev. Neurobiol. 67, 1204–1215 (2007).
Hadaczek, P. et al. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2–hAADC. Mol. Ther. 18, 1458–1461 (2010).
Forsayeth, J. R. et al. A dose-ranging study of AAV–hAADC therapy in Parkinsonian monkeys. Mol. Ther. 14, 571–577 (2006).
Sanftner, L. M. et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp. Neurol. 194, 476–483 (2005).
Tenenbaum, L. et al. Recombinant AAV-mediated gene delivery to the central nervous system. J. Gene Med. 6 (Suppl. 1), 212–222 (2004).
Graham, L. D. Ecdysone-controlled expression of transgenes. Expert Opin. Biol. Ther. 2, 525–535 (2002).
Blesch, A. et al. Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol. Ther. 11, 916–925 (2005).
Acknowledgements
This work is supported by the National Institutes of Health grant AG10435, the Veterans Administration, the Alzheimer's Association, the Shiley Family Foundation, and the Dr Miriam and Sheldon G. Adelson Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Nagahara, A., Tuszynski, M. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10, 209–219 (2011). https://doi.org/10.1038/nrd3366
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3366
This article is cited by
-
Targeting synapse function and loss for treatment of neurodegenerative diseases
Nature Reviews Drug Discovery (2024)
-
Effect of Cyclosporin H on ischemic injury and neutrophil infiltration in cerebral infarct model of rats via PET imaging
Annals of Nuclear Medicine (2024)
-
Neurotrophin growth factors and their receptors as promising blood biomarkers for Alzheimer’s Disease: a gene expression analysis study
Molecular Biology Reports (2024)
-
Neural stem/progenitor cell therapy for Alzheimer disease in preclinical rodent models: a systematic review and meta-analysis
Stem Cell Research & Therapy (2023)
-
Loss of MBNL1-mediated retrograde BDNF signaling in the myotonic dystrophy brain
Acta Neuropathologica Communications (2023)