Abstract
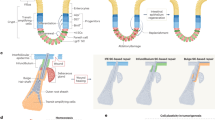
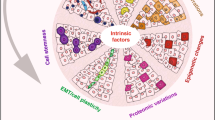
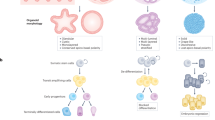
Differentiation therapies that induce malignant cells to stop growing and revert to normal tissue-specific differentiated cell types are successful in the treatment of a few specific haematological tumours. However, this approach has not been widely applied to solid tumours because their developmental origins are less well understood. Recent advances suggest that understanding tumour cell plasticity and how intrinsic factors (such as genetic noise and microenvironmental signals, including physical cues from the extracellular matrix) govern cell state switches will help in the development of clinically relevant differentiation therapies for solid cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yamanaka, S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 41 (Suppl. 1), 51–56 (2008).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Swanton, C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 72, 4875–4882 (2012).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Navin, N. et al. Inferring tumor progression from genomic heterogeneity. Genome Res. 20, 68–80 (2010).
Calbo, J. et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 19, 244–256 (2011).
Celia-Terrassa, T. et al. Epithelial–mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J. Clin. Invest. 122, 1849–1868 (2012).
Tsuji, T., Ibaragi, S. & Hu, G. F. Epithelial–mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 69, 7135–7139 (2009).
Mroz, E. A. et al. High intratumor genetic heterogeneity is related to worse outcome in patients with head and neck squamous cell carcinoma. Cancer 119, 3034–3042 (2013).
Merlo, L. M. et al. A comprehensive survey of clonal diversity measures in Barrett's esophagus as biomarkers of progression to esophageal adenocarcinoma. Cancer Prev. Res. 3, 1388–1397 (2010).
Marusyk, A., Almendro, V. & Polyak, K. Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer 12, 323–334 (2012).
Polyak, K. Heterogeneity in breast cancer. J. Clin. Invest. 121, 3786–3788 (2011).
Bedard, P. L., Hansen, A. R., Ratain, M. J. & Siu, L. L. Tumour heterogeneity in the clinic. Nature 501, 355–364 (2013).
Gupta, P. B. et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 146, 633–644 (2011).
Pisco, A. O. et al. Non-Darwinian dynamics in therapy-induced cancer drug resistance. Nat. Commun. 4, 2467 (2013).
Huang, S., Eichler, G., Bar-Yam, Y. & Ingber, D. E. Cell fates as high-dimensional attractor states of a complex gene regulatory network. Phys. Rev. Lett. 94, 128701 (2005).
Chang, H. H., Hemberg, M., Barahona, M., Ingber, D. E. & Huang, S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 453, 544–547 (2008).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Pierce, G. B. & Wallace, C. Differentiation of malignant to benign cells. Cancer Res. 31, 127–134 (1971).
Lee, G. Y. et al. Stochastic acquisition of a stem cell-like state and drug tolerance in leukemia cells stressed by radiation. Int. J. Hematol. 93, 27–35 (2011).
Charles, N. et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 6, 141–152 (2010).
Chaffer, C. L. et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl Acad. Sci. USA 108, 7950–7955 (2011).
Hoek, K. S. & Goding, C. R. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 23, 746–759 (2010).
Roesch, A. et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 141, 583–594 (2010).
Almendro, V. et al. Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell Rep. 6, 514–527 (2014).
Guo, Y., Eichler, G. S., Feng, Y., Ingber, D. E. & Huang, S. Towards a holistic, yet gene-centered analysis of gene expression profiles: a case study of human lung cancers. J. Biomed. Biotechnol. 2006, 69141 (2006).
Huang, S. & Ingber, D. E. Shape-dependent control of cell growth, differentiation, and apoptosis: switching between attractors in cell regulatory networks. Exp. Cell Res. 261, 91–103 (2000).
Huang, S. & Ingber, D. E. A non-genetic basis for cancer progression and metastasis: self-organizing attractors in cell regulatory networks. Breast Dis. 26, 27–54 (2006).
Kauffman, S. Differentiation of malignant to benign cells. J. Theor. Biol. 31, 429–451 (1971).
Brock, A., Chang, H. & Huang, S. Non-genetic heterogeneity — a mutation-independent driving force for the somatic evolution of tumours. Nat. Rev. Genet. 10, 336–342 (2009).
Flusberg, D. A., Roux, J., Spencer, S. L. & Sorger, P. K. Cells surviving fractional killing by TRAIL exhibit transient but sustainable resistance and inflammatory phenotypes. Mol. Biol. Cell 24, 2186–2200 (2013).
Spencer, S. L., Gaudet, S., Albeck, J. G., Burke, J. M. & Sorger, P. K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432 (2009).
Ingber, D. E. Tensegrity, I. I. How structural networks influence cellular information processing networks. J. Cell Sci. 116, 1397–1408 (2003).
Kauffman, S. Homeostasis and differentiation in random genetic control networks. Nature 224, 177–178 (1969).
Waddington, C. The Strategy of the Genes (Allen and Unwin, 1957).
Hoffmann, M. et al. Noise-driven stem cell and progenitor population dynamics. PLoS ONE 3, e2922 (2008).
Werfel, J. et al. How changes in extracellular matrix mechanics and gene expression variability might combine to drive cancer progression. PLoS ONE 8, e76122 (2013).
Kauffman, S. A. The Origins of Order (Oxford Univ. Press, 1993).
Huang, S. & Ingber, D. E. A discrete cell cycle checkpoint in late G(1) that is cytoskeleton-dependent and MAP kinase (Erk)-independent. Exp. Cell Res. 275, 255–264 (2002).
Murphy, D. Gene expression studies using microarrays: principles, problems, and prospects. Adv. Physiol. Educ. 26, 256–270 (2002).
Brock, A., Huang, S. & Ingber, D. E. Identification of a distinct class of cytoskeleton-associated mRNAs using microarray technology. BMC Cell Biol. 4, 6 (2003).
Ohnishi, K. et al. Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell 156, 663–677 (2014).
Ma, H. et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 511, 177–183 (2014).
Hanash, K. A. Metastatic tumors to the testicles. Prog. Clin. Biol. Res. 203, 61–67 (1985).
Ohm, J. E. et al. Cancer-related epigenome changes associated with reprogramming to induced pluripotent stem cells. Cancer Res. 70, 7662–7673 (2010).
Brock, A. et al. Cellular reprogramming: a new technology frontier in pharmaceutical research. Pharm. Res. 29, 35–52 (2012).
West-Eberhard, M. J. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102 (Suppl. 1), 6543–6549 (2005).
Schedin, P. & Keely, P. J. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 3, a003228 (2011).
Ingber, D. E. Can cancer be reversed by engineering the tumor microenvironment? Semin. Cancer Biol. 18, 356–364 (2008).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Vidal, M., Larson, D. E. & Cagan, R. L. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev. Cell 10, 33–44 (2006).
Vidal, M. et al. A role for the epithelial microenvironment at tumor boundaries: evidence from Drosophila and human squamous cell carcinomas. Am. J. Pathol. 176, 3007–3014 (2010).
Leung, C. T. & Brugge, J. S. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482, 410–413 (2012).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Barcellos-Hoff, M. H. & Ravani, S. A. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60, 1254–1260 (2000).
Maffini, M. V., Soto, A. M., Calabro, J. M., Ucci, A. A. & Sonnenschein, C. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 117, 1495–1502 (2004).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007).
Jamieson, J. D. et al. Cell surface properties of normal, differentiating, and neoplastic pancreatic acinar cells. Cancer 47, 1516–1527 (1981).
Sternlicht, M. D. et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98, 137–146 (1999).
Boyd, N. et al. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 10, 569–580 (2009).
dos Santos Silva, I. et al. The insulin-like growth factor system and mammographic features in premenopausal and postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 15, 449–455 (2006).
Provenzano, P. P. et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 11 (2008).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Mouw, J. K. et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med. 20, 360–367 (2014).
Liu, S. et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2, 78–91 (2014).
Conley, S. J. et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc. Natl Acad. Sci. USA 109, 2784–2789 (2012).
Biddle, A. et al. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 71, 5317–5326 (2011).
Supernat, A. et al. Epithelial–mesenchymal transition and cancer stem cells in endometrial cancer. Anticancer Res. 33, 5461–5469 (2013).
Korpal, M. et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 17, 1101–1108 (2011).
Thompson, E. W. & Haviv, I. The social aspects of EMT–MET plasticity. Nat. Med. 17, 1048–1049 (2011).
Goel, S., Wong, A. H. & Jain, R. K. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb. Perspect. Med. 2, a006486 (2012).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Beck, B. et al. A vascular niche and a VEGF–Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478, 399–403 (2011).
Cao, Z. et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell 25, 350–365 (2014).
Braun, A. C. & Wood, H. N. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc. Natl Acad. Sci. USA 73, 496–500 (1976).
Rangecroft, L., Lauder, I. & Wagget, J. Spontaneous maturation of stage IV–S neuroblastoma. Arch. Dis. Child 53, 815–817 (1978).
Stewart, T. A. & Mintz, B. Successive generations of mice produced from an established culture line of euploid teratocarcinoma cells. Proc. Natl Acad. Sci. USA 78, 6314–6318 (1981).
Mintz, B. & Illmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl Acad. Sci. USA 72, 3585–3589 (1975).
Coleman, W. B., Wennerberg, A. E., Smith, G. J. & Grisham, J. W. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am. J. Pathol. 142, 1373–1382 (1993).
McCullough, K. D., Coleman, W. B., Smith, G. J. & Grisham, J. W. Age-dependent induction of hepatic tumor regression by the tissue microenvironment after transplantation of neoplastically transformed rat liver epithelial cells into the liver. Cancer Res. 57, 1807–1813 (1997).
McCullough, K. D. et al. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc. Natl Acad. Sci. USA 95, 15333–15338 (1998).
Booth, B. W., Boulanger, C. A., Anderson, L. H. & Smith, G. H. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene 30, 679–689 (2011).
DeCosse, J. J., Gossens, C. L., Kuzma, J. F. & Unsworth, B. R. Breast cancer: induction of differentiation by embryonic tissue. Science 181, 1057–1058 (1973).
Wong, Y. C., Cunha, G. R. & Hayashi, N. Effects of mesenchyme of the embryonic urogenital sinus and neonatal seminal vesicle on the cytodifferentiation of the Dunning tumor: ultrastructural study. Acta Anat. 143, 139–150 (1992).
Chung, L. W., Zhau, H. E. & Ro, J. Y. Morphologic and biochemical alterations in rat prostatic tumors induced by fetal urogenital sinus mesenchyme. Prostate 17, 165–174 (1990).
Watanabe, T. K., Hansen, L. J., Reddy, N. K., Kanwar, Y. S. & Reddy, J. K. Differentiation of pancreatic acinar carcinoma cells cultured on rat testicular seminiferous tubular basement membranes. Cancer Res. 44, 5361–5368 (1984).
Cunha, G. R. et al. Epithelial–mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J. Cell Biol. 96, 1662–1670 (1983).
Hendrix, M. J. et al. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer 7, 246–255 (2007).
Kenny, P. A. & Bissell, M. J. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int. J. Cancer 107, 688–695 (2003).
Xie, J. W. & Haslam, S. Z. Extracellular matrix, Rac1 signaling, and estrogen-induced proliferation in MCF-7 breast cancer cells. Breast Cancer Res. Treat. 110, 257–268 (2008).
Neubauer, H. et al. A laminin-rich basement membrane matrix influences estrogen receptor β expression and morphology of MDA-MB-231 breast cancer cells. Oncol. Rep. 21, 475–481 (2009).
Weaver, V. M. et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 (1997).
McKinnell, R. G., Deggins, B. A. & Labat, D. D. Transplantation of pluripotential nuclei from triploid frog tumors. Science 165, 394–396 (1969).
Dolberg, D. S. & Bissell, M. J. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature 309, 552–556 (1984).
Lee, L. M., Seftor, E. A., Bonde, G., Cornell, R. A. & Hendrix, M. J. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. 233, 1560–1570 (2005).
Topczewska, J. M. et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat. Med. 12, 925–932 (2006).
Kulesa, P. M. et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl Acad. Sci. USA 103, 3752–3757 (2006).
Shackleton, M., Quintana, E., Fearon, E. R. & Morrison, S. J. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 138, 822–829 (2009).
Matthay, K. K. et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N. Engl. J. Med. 341, 1165–1173 (1999).
Weintraub, H. et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251, 761–766 (1991).
Nerlov, C., Querfurth, E., Kulessa, H. & Graf, T. GATA-1 interacts with the myeloid PU.1 transcription factor and represses PU.1-dependent transcription. Blood 95, 2543–2551 (2000).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D. A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 (2008).
Mack, S. C. et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 506, 445–450 (2014).
Lee, R. S. et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J. Clin. Invest. 122, 2983–2988 (2012).
Molenaar, J. J. et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483, 589–593 (2012).
Rausch, T. et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 148, 59–71 (2012).
Shlush, L. I. et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506, 328–333 (2014).
Parker, M. et al. C11orf95–RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 506, 451–455 (2014).
Lewis, P. W. et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340, 857–861 (2013).
Fang, H. & Declerck, Y. A. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 73, 4965–4977 (2013).
Neesse, A. et al. Stromal biology and therapy in pancreatic cancer. Gut 60, 861–868 (2011).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Salmon, H. et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910 (2012).
Ozdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014).
Xiao, Q. & Ge, G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron. 5, 261–273 (2012).
Fingleton, B. Matrix metalloproteinases: roles in cancer and metastasis. Front. Biosci. 11, 479–491 (2006).
Bischof, A. G. et al. Breast cancer normalization induced by embryonic mesenchyme is mediated by extracellular matrix biglycan. Integr. Biol. 5, 1045–1056 (2013).
Heinaniemi, M. et al. Gene-pair expression signatures reveal lineage control. Nat. Methods 10, 577–583 (2013).
di Bernardo, D. et al. Chemogenomic profiling on a genome-wide scale using reverse-engineered gene networks. Nat. Biotech. 23, 377–383 (2005).
Brock, A. et al. Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice. Sci. Transl Med. 6, 217ra2 (2014).
Ergun, A., Lawrence, C. A., Kohanski, M. A., Brennan, T. A. & Collins, J. J. A network biology approach to prostate cancer. Mol. Syst. Biol. 3, 82 (2007).
Huang, S. The molecular and mathematical basis of Waddington's epigenetic landscape: a framework for post-Darwinian biology? Bioessays 34, 149–157 (2012).
Xing, F., Saidou, J. & Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. (Landmark Ed.) 15, 166–179 (2010).
De Wever, O., Demetter, P., Mareel, M. & Bracke, M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 123, 2229–2238 (2008).
Cucina, A. et al. Zebrafish embryo proteins induce apoptosis in human colon cancer cells (Caco2). Apoptosis 11, 1617–1628 (2006).
Allegrucci, C. et al. Epigenetic reprogramming of breast cancer cells with oocyte extracts. Mol. Cancer 10, 7 (2011).
Cooper, M. & Pinkus, H. Intrauterine transplantation of rat basal cell carcinoma as a model for reconversion of malignant to benign growth. Cancer Res. 37, 2544–2552 (1977).
Krause, S., Maffini, M. V., Soto, A. M. & Sonnenschein, C. The microenvironment determines the breast cancer cells' phenotype: organization of MCF7 cells in 3D cultures. BMC Cancer 10, 263 (2010).
Trimboli, A. J. et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461, 1084–1091 (2009).
Maffini, M. V., Calabro, J. M., Soto, A. M. & Sonnenschein, C. Stromal regulation of neoplastic development: age-dependent normalization of neoplastic mammary cells by mammary stroma. Am. J. Pathol. 167, 1405–1410 (2005).
Soda, Y. et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl Acad. Sci. USA 108, 4274–4280 (2011).
Breitman, T. R., Selonick, S. E. & Collins, S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl Acad. Sci. USA 77, 2936–2940 (1980).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Kang, Y. & Massague, J. Epithelial–mesenchymal transitions: twist in development and metastasis. Cell 118, 277–279 (2004).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Acknowledgements
The authors thank K. Johnson of Boston Children's Hospital for assistance with figure preparation. This work was supported by a Breast Cancer Research Foundation Translational Research Grant (14-60-26-BROC) to A.B., and a Department of Defense Breast Cancer Innovator Award (BC074986) to D.E.I. The authors apologize to the many people whose work we could not cite owing to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.K. holds equity in Momenta Pharmaceuticals and D.E.I. consults to Momenta Pharmaceuticals, SynDevRx Inc. and Celgene Cellular Therapeutics, on work unrelated to the material included here. A.B. declares no competing interests as defined by Nature Publishing Group or other interests that might be perceived to influence the interpretation of the article.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Ongoing clinical trials phase I to III targeting tumor microenvironment (interventional) (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Brock, A., Krause, S. & Ingber, D. Control of cancer formation by intrinsic genetic noise and microenvironmental cues. Nat Rev Cancer 15, 499–509 (2015). https://doi.org/10.1038/nrc3959
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3959
This article is cited by
-
Critical transition and reversion of tumorigenesis
Experimental & Molecular Medicine (2023)
-
Emerging role of tumor cell plasticity in modifying therapeutic response
Signal Transduction and Targeted Therapy (2020)
-
Defining rules for cancer cell proliferation in TRAIL stimulation
npj Systems Biology and Applications (2019)
-
A multi-state model of chemoresistance to characterize phenotypic dynamics in breast cancer
Scientific Reports (2018)
-
Regulation of genome organization and gene expression by nuclear mechanotransduction
Nature Reviews Molecular Cell Biology (2017)